The electronic configuration of elements is based on the arrangement of electrons in shells or energy levels around the nucleus. Here are the electronic configurations for elements 1 to 30 (Hydrogen to Zinc):
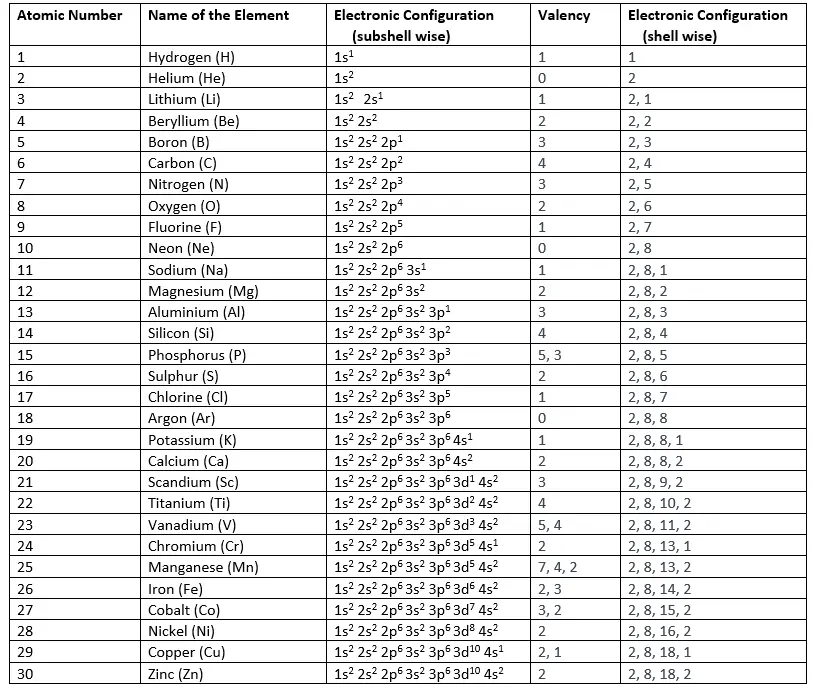
Remember, the electronic configurations of some elements (like Chromium and Copper) are exceptions to the general rules due to the extra stability provided by half-filled or fully filled subshells.
Frequently Asked Questions
- Why do Chromium and Copper have unusual electronic configurations?
Answer: Chromium and Copper have unusual electronic configurations due to the extra stability provided by half-filled (in the case of Chromium) and fully filled (in the case of Copper) d-subshells. Chromium has the electronic configuration of 1s² 2s² 2p? 3s² 3p? 4s¹ 3d?, and Copper has 1s² 2s² 2p? 3s² 3p? 4s¹ 3d¹?. This is because a half-filled or fully filled d-subshell is more stable. - What is the significance of the Aufbau principle in determining electronic configuration?
Answer: The Aufbau principle states that electrons fill orbitals starting with the lowest energy level before moving to higher levels. This principle helps in predicting the electronic configurations of elements by filling electrons into atomic orbitals in the order of increasing energy levels. - Can the electronic configuration determine the chemical properties of an element?
Answer: Yes, the electronic configuration of an element plays a crucial role in determining its chemical properties, including valency, the type of bonds it forms, its reactivity, and placement in the periodic table. - Why is it important to learn the electronic configurations of elements?
Answer: Understanding the electronic configurations of elements is essential for grasping concepts in chemistry such as bonding, reactivity, periodic trends, and the behavior of elements in various chemical reactions. - How does the electronic configuration change across a period in the periodic table?
Answer: Across a period in the periodic table, the principal quantum number remains the same, but electrons are added to the same outermost shell. This results in a gradual increase in the number of electrons in the valence shell as you move from left to right across a period. - What is the role of valence electrons in chemical bonding?
Answer: Valence electrons, which are the electrons in the outermost shell of an atom, play a key role in chemical bonding. They are the electrons involved in forming bonds with other atoms. - Why are noble gases chemically inert?
Answer: Noble gases are chemically inert because they have a complete valence shell, typically with an s²p? configuration (except for Helium, which is 1s²). This full valence shell makes them energetically stable and unreactive. - How does the electronic configuration affect the atomic size of elements?
Answer: Atomic size generally decreases across a period due to the increase in nuclear charge, which pulls the electrons closer to the nucleus. In contrast, atomic size increases down a group as additional electron shells are added, increasing the distance between the outermost electrons and the nucleus.
Multiple Choice Questions
- What is the electronic configuration of Chromium (Cr, atomic number 24)?
A) 1s² 2s² 2p? 3s² 3p? 4s² 3d?
B) 1s² 2s² 2p? 3s² 3p? 4s¹ 3d?
C) 1s² 2s² 2p? 3s² 3p? 4s² 3d?
D) 1s² 2s² 2p? 3s² 3p? 4s¹ 3d? - Which principle explains the order in which electrons fill atomic orbitals?
A) Hund’s Rule
B) Pauli Exclusion Principle
C) Aufbau Principle
D) Heisenberg Uncertainty Principle - Which element has the electronic configuration 1s² 2s² 2p? 3s² 3p??
A) Chlorine (Cl)
B) Sulfur (S)
C) Argon (Ar)
D) Phosphorus (P) - Why are noble gases considered chemically inert?
A) They have a complete d-subshell.
B) They have a complete valence shell.
C) They have no valence electrons.
D) They have a high electronegativity. - What is the maximum number of electrons present on the d-orbital when writing the electronic configuration of any element??
A) 2
B) 6
C) 8
D) 10 - Which of the following elements has a half-filled p-subshell?
A) Carbon (C)
B) Nitrogen (N)
C) Oxygen (O)
D) Boron (B) - The electronic configuration 1s² 2s² 2p? 3s² 3p? 4s¹ corresponds to which element?
A) Potassium (K)
B) Calcium (Ca)
C) Scandium (Sc)
D) Argon (Ar) - Which element has the electronic configuration 1s² 2s² 2p¹?
A) Lithium (Li)
B) Beryllium (Be)
C) Boron (B)
D) Carbon (C) - What is the significance of valence electrons in chemical bonding?
A) They determine the atomic mass.
B) They are involved in forming chemical bonds.
C) They are always in the d-subshell.
D) They are found in the innermost shell. - How many unpaired electrons are present in fluorine atoms ‘F’??
A) 1
B) 2
C) 0
D) 3
Answers:
- B) 1s² 2s² 2p? 3s² 3p? 4s¹ 3d?
- C) Aufbau Principle
- A) Chlorine (Cl)
- B) They have a complete valence shell.
- D)
- A) Carbon (C)
- A) Potassium (K)
- C) Boron (B)
- B) They are involved in forming chemical bonds.
- A)
 Skip to content
Skip to content