Activity 1.5 Class 10 Science: Ferrous Sulphate Heating
Introduction to Activity 1.5 for class 10 science
Activity 1.5 in Class 10 Science NCERT book, Chapter 1: Chemical Reactions and Equations, is an experiment that helps students understand the concept of chemical reactions by observing the changes that occur when ferrous sulphate crystals are heated. This article will provide a brief theory behind this activity, describe the procedure, and answer the question asked in the text.
The Theory Behind Activity 1.5
The experiment demonstrates a type of chemical reaction called thermal decomposition. When certain compounds are heated, they may break down into two or more simpler substances. In this case, ferrous sulphate $(FeSO_4)$ undergoes a chemical reaction when heated, resulting in a change in its physical and chemical properties.
Materials Required for Activity 1.5
- Ferrous sulphate crystals (approximately 2 g)
- A dry boiling tube
- A burner or spirit lamp
- A tripod stand or test tube holder to hold the boiling tube
- Heat-resistant gloves or tongs (for safety)
Procedure for Activity 1.5
- Gather materials:
To perform this experiment, you will need approximately $2 g$ of ferrous sulphate crystals, a dry boiling tube, a burner or spirit lamp, and a tripod stand to hold the boiling tube. - Note the color of ferrous sulphate crystals:
Before starting the experiment, note the color of the ferrous sulphate crystals. This is important for comparing the changes that occur after heating. - Heat the boiling tube:
Place the ferrous sulphate crystals in the dry boiling tube. Heat the boiling tube over the flame of the burner or spirit lamp, following the correct heating procedure as shown in Figure given below. - Observe the color change:
Watch the crystals carefully as they are heated. Record any changes in the color and appearance of the crystals.
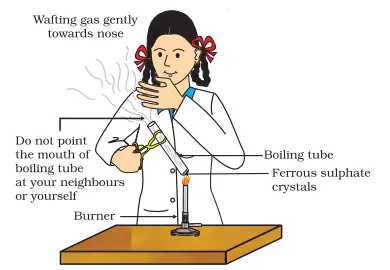
Observations and Results
Upon heating, the green-colored ferrous sulphate crystals change to a reddish-brown color. This color change signifies a chemical reaction taking place. The reaction can be represented as follows:
$FeSO_4 (s) \rightarrow Fe_2O_3 (s) + SO_2 (g) + SO_3 (g)$
The green ferrous sulphate crystals (FeSO4) decompose into reddish-brown ferric oxide $(Fe2O_3)$, sulfur dioxide gas $(SO_2)$, and sulfur trioxide gas $(SO_3)$ upon heating.
Conclusion
Activity 1.5 from Class 10 Science NCERT book demonstrates the thermal decomposition of ferrous sulphate crystals. Students observe a color change and the formation of new substances as the crystals are heated, providing a practical understanding of chemical reactions.
 Skip to content
Skip to content
It helped a lot for understanding the topics
Thanks for taking the time to visit. We’re happy it was helpful.