Activity 1.8 in Class 10 Science NCERT Book
Introduction
In this article, we will discuss Activity 1.8 from Chapter 1: Chemical Reactions and Equations in the Class 10 Science ( ncert notes class 10 science ) NCERT textbook.
This activity focuses on the fascinating colour change that occurs in silver chloride when exposed to sunlight and is an excellent example of a photochemical decomposition reaction.
We will take a look into the aim, theory, procedure, observation, and conclusion of Activity 1.8 in this article.
Aim/Objective of Activity 1.8:
Activity 1.8 aims to help students observe and understand the phenomenon of a photochemical decomposition reaction through the change in colour of silver chloride when placed in sunlight. This activity reinforces the knowledge of chemical reactions and their various types.
Materials and Apparatus Required:
- Approximately 2 g of silver chloride (AgCl)
- A china dish
- Sunlight
Procedure:
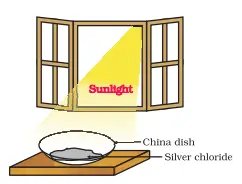
Step 1: Observe the initial colour Observe the colour of silver chloride. It appears as a white crystalline solid.
Step 2: Place silver chloride in sunlight Place approximately 2 g of silver chloride in the china dish. Next, put the china dish in the sunlight for some time.
Step 3: Observe the colour change After a while, notice that the colour of the silver chloride changes from its original white colour to grey.
Explanation of Activity 1.8:
The change in colour observed in Activity 1.8 is due to a photochemical decomposition reaction. This type of reaction occurs when a chemical compound breaks down into simpler substances when exposed to light.
Silver chloride, a white crystalline solid, decomposes into silver metal $(Ag)$ and chlorine gas $(Cl_2)$ when exposed to sunlight. The silver metal that forms in this reaction is grey, which is responsible for the observed colour change.
The balanced chemical equation for this reaction is as follows:
$2AgCl(s) \rightarrow 2Ag(s) + Cl_2(g)$
Here, $’s’$ denotes a solid state and $’g’$ represents a gaseous state.
The light energy provided by the sun acts as a catalyst, facilitating the decomposition of silver chloride. This process is also known as photodecomposition, and it is a type of chemical reaction where light energy is absorbed by the reactants to facilitate the reaction.
Conclusion:
Activity 1.8 in Class 10 Science’s Chapter 1 demonstrates the concept of photochemical decomposition reaction through the colour change in silver chloride when exposed to sunlight.
This experiment not only reinforces the knowledge of various types of chemical reactions but also provides an opportunity for students to engage in hands-on learning. By understanding the process behind this intriguing colour change, students can better appreciate the intricacies of chemical reactions and their applications in everyday life.
Frequently asked questions
What is the balanced chemical equation for the decomposition reaction of silver chloride in the presence of sunlight?
The balanced chemical equation for the decomposition reaction of silver chloride in the presence of sunlight is:
$2AgCl(s) \rightarrow 2Ag(s) + Cl_2(g)$
What role does sunlight play in the decomposition reaction of silver chloride?
Sunlight, particularly UV light, provides the energy required to initiate the decomposition reaction of silver chloride. The photons in sunlight break the bonds within the silver chloride, causing it to decompose into its constituent elements, silver and chlorine.
Why does the colour of silver chloride change during the decomposition reaction?
The colour of silver chloride changes during the decomposition reaction because it breaks down into silver and chlorine. Silver chloride is initially a white crystalline solid, and as it decomposes, the formation of silver metal gives it a grey appearance.
What are the products formed when silver chloride undergoes a decomposition reaction?
The products formed when silver chloride undergoes a decomposition reaction is silver (Ag) and chlorine (Cl?).
How can the concept of photolysis be applied to explain the reaction of silver chloride exposed to sunlight?
Photolysis is a process in which a chemical compound is broken down by photons (light energy). In the case of silver chloride, sunlight provides the photons required to break its bonds, resulting in the decomposition of the compound into silver and chlorine. This is an example of photolysis applied to explain the reaction of silver chloride exposed to sunlight.
 Skip to content
Skip to content