Activity 1.10 Class 10 Science NCERT Chapter 1
Introduction
Activity 1.10 in the Class 10 Science NCERT book, Chapter 1: Chemical Reactions and Equations, is an excellent way to understand the concept of double displacement reactions. This activity provides a hands-on experience of a chemical reaction involving two aqueous solutions and the formation of a precipitate. Let’s dive into the details of this experiment.
Objective
The objective of Activity 1.10 class 10 science is to demonstrate a double displacement reaction between sodium sulphate and barium chloride, resulting in the formation of barium sulphate and sodium chloride.
Materials Required
- Sodium sulphate solution (3 mL)
- Barium chloride solution (3 mL)
- Two test tubes
- Test tube holder
- Dropper
- Stirring rod
Procedure
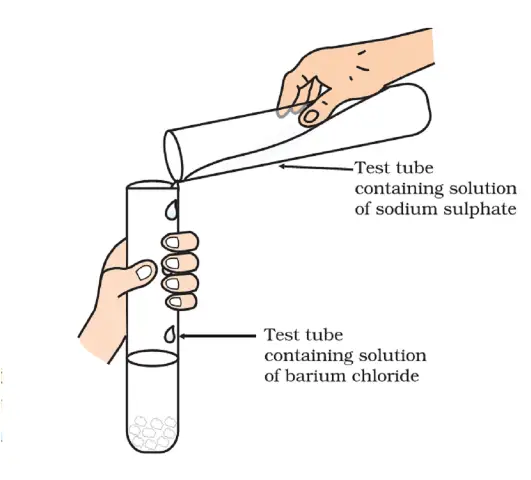
- Take about 3 mL of sodium sulphate solution in a test tube. Label it as ‘A.’
- In another test tube, take about 3 mL of barium chloride solution. Label it as ‘B.’
- Mix the two solutions by carefully pouring the contents of test tube A into test tube B (Figure:- Formation of barium sulphate and sodium chloride).
- Gently stir the mixture using a stirring rod.
Observation
On mixing the sodium sulphate solution and the barium chloride solution, you will observe that a white precipitate is formed in the test tube. This precipitate is the result of a chemical reaction between the two solutions, indicating a double displacement reaction.
Result and Conclusion
The reaction that takes place in this activity is a double displacement reaction, wherein the ions of two compounds exchange places in an aqueous solution to form two new compounds. In this case, sodium sulphate and barium chloride react to form barium sulphate and sodium chloride. The chemical equation for this reaction is:
$Na_2SO_4(aq) + BaCl_2(aq) \rightarrow BaSO_4(s) + 2NaCl(aq)$
The white precipitate formed in the reaction is barium sulphate $(BaSO_4)$, which is insoluble in water, while sodium chloride $(NaCl)$ remains dissolved in the solution.
Through Activity 1.10 of class 10 science, students can visually observe a double displacement reaction and understand the concept of precipitation in a chemical reaction.
Activity-based questions for Activity 1.10 Class 10 Science
Very Short Answer Type Questions
- What type of reaction occurs when sodium sulphate and barium chloride solutions are mixed?
Answer: Double displacement reaction. - What is the white solid formed when sodium sulphate and barium chloride solutions react?
Answer: Barium sulphate $(BaSO_4)$. - Write the balanced chemical equation for the reaction between sodium sulphate and barium chloride.
Answer: $Na_2SO_4(aq) + BaCl_2(aq) \rightarrow BaSO_4(s) + 2NaCl(aq)$ - Which compound remains dissolved in the solution after sodium sulphate reacts with barium chloride?
Answer: Sodium chloride (NaCl). - Why is stirring necessary during this reaction?
Answer: Stirring helps mix the two solutions and facilitates the reaction.
Conceptual Questions
- Why is the reaction between sodium sulphate and barium chloride classified as a double displacement reaction?
Answer: The reaction is classified as a double displacement reaction because the ions of the two compounds exchange places to form two new compounds (barium sulphate and sodium chloride). - What is a precipitate, and how does it form during the reaction between sodium sulphate and barium chloride?
Answer: A precipitate is an insoluble solid that forms during a chemical reaction. In this case, the precipitate is barium sulphate (BaSO4), which forms due to the double displacement reaction between sodium sulphate and barium chloride. - What is the significance of a balanced chemical equation?
Answer: A balanced chemical equation represents the conservation of mass during a chemical reaction. It ensures that the number of atoms for each element on the reactant side is equal to the number of atoms for each element on the product side.
Multiple Choice Questions
- Which of the following is the balanced chemical equation for the reaction between sodium sulphate and barium chloride?
a) $Na_2SO_4(aq) + BaCl_2(aq) \rightarrow BaSO_4(s) + NaCl(aq)$
b) $Na_2SO_4(aq) + BaCl_2(aq) \rightarrow BaSO_4(s) + 2NaCl(aq)$
c) $2Na_2SO_4(aq) + BaCl_2(aq) \rightarrow BaSO_4(s) + 4NaCl(aq)$
d) $Na_2SO_4(aq) + 2BaCl_2(aq) \rightarrow BaSO_4(s) + 2NaCl(aq)$
Answer: b) $Na_2SO_4(aq) + BaCl_2(aq) \rightarrow BaSO_4(s) + 2NaCl(aq)$ - Which type of reaction is characterized by the exchange of ions between two compounds in an aqueous solution?
a) Single displacement
b) Double displacement
c) Combination
d) Decomposition
Answer: b) Double displacement - What is the precipitate formed during the reaction between sodium sulphate and barium chloride?
a) Sodium chloride
b) Sodium sulphate
c) Barium chloride
d) Barium sulphate
Answer: d) Barium sulphate - In the reaction between sodium sulphate and barium chloride, which compound remains dissolved in the solution?
a) Sodium sulphate
b) Sodium chloride
c) Barium sulphate
d) Barium chloride
Answer: b) Sodium chloride - What is the main purpose of using a stirring rod during the reaction between sodium sulphate and barium chloride?
a) To heat the solution
b) To measure the temperature
c) To mix the solutions and facilitate the reaction
d) To remove the precipitate
Answer: c) To mix the solutions and facilitate the reaction