Exploring Chemical Reactions through Activity 1.9 Class 10 Science
The objective of Activity 1.9 :
The main objective of this activity 1.9 class 10 science is to enable students to observe and understand the chemical reaction that takes place between iron nails and copper sulphate solution.
Materials Required:
- Three clean iron nails
- Sandpaper
- Two test tubes marked (A) and (B)
- 20 mL copper sulphate solution
- A thread
Procedure:
Step 1: Preparation of Iron Nails
Start by cleaning the three iron nails with sandpaper to remove any rust or impurities from their surfaces.
Step 2: Preparation of Test Tubes
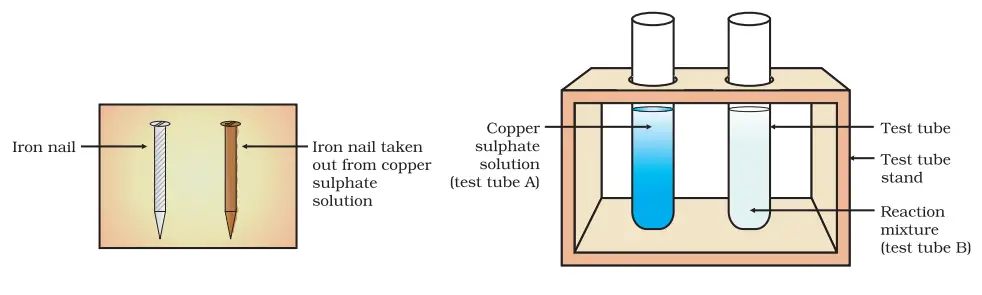
Fill both test tubes (A) and (B) with 10 mL of copper sulphate solution each.
Step 3: Immersion of Iron Nails in Test Tube B
Tie two iron nails together with thread and place them in test tube B, making sure they are completely submerged in the copper sulphate solution. Leave them there for about 20 minutes.
Step 4: Observation and Comparison
Remove the iron nails from test tube B after 20 minutes and compare the intensity of the blue colour of the copper sulphate solution in both test tubes (A) and (B). Compare the colours of the iron nails that were immersed in the solution to those that were set aside.
Analysis and Conclusion:
When compared to the solution in test tube A, you will note that the blue tint of the copper sulphate solution in test tube B has become lighter. In addition, the iron nails dipped in the solution have turned reddish, indicating copper deposition.
This colour change indicates that a chemical reaction occurred between the iron nails and the copper sulphate solution. The following equation can be used to illustrate the reaction:
$Fe(s) + CuSO_4(aq) \rightarrow FeSO_4(aq) + Cu(s)$
The iron $(Fe)$ has displaced copper $(Cu)$ from the copper sulphate solution, resulting in the formation of ferrous sulphate $(FeSO_4)$ and the deposition of copper on the iron nails.
In conclusion, Activity 1.9 effectively demonstrates the concept of chemical reactions and displacement reactions to students, enabling them to observe the changes that occur when two different elements interact with each other.
1.9 activity-based question
Here are some activity-based questions based on this class 10 science NCERT book chapter 1 concepts.
Conceptual Questions:
Question: What type of chemical reaction is demonstrated by the interaction between iron nails and copper sulphate solution?
Answer: The reaction between iron nails and copper sulphate solution is an example of a displacement reaction. In this case, the iron displaces copper from the copper sulphate solution, resulting in the formation of ferrous sulphate and the deposition of copper on the iron nails.
Question: Why is it important to clean the iron nails with sandpaper before conducting the experiment?
Answer: Cleaning the iron nails with sandpaper is important to remove any rust or impurities from their surfaces. This ensures that the observed chemical reaction is purely between the iron and the copper sulphate solution, without any interference from other substances that might be present on the nails.
Question: What is the significance of comparing the colour of the copper sulphate solutions in test tubes A and B after the experiment?
Answer: Comparing the colour of the copper sulphate solutions in test tubes A and B helps to highlight the chemical changes that have taken place during the experiment. The lighter blue color in test tube B indicates that a chemical reaction has occurred, resulting in the formation of ferrous sulphate and the deposition of copper on the iron nails.
Multiple Choice Questions:
Question 1. What type of chemical reaction takes place when iron nails are immersed in copper sulphate solution?
a) Combination reaction
b) Decomposition reaction
c) Displacement reaction
d) Double displacement reaction
Answer: c) Displacement reaction
Question 2. What is the chemical formula for copper sulphate?
a) CuSO3
b) CuSO4
c) CuS
d) Cu2S
Answer: b) CuSO4
Question 3. After the reaction between iron nails and copper sulphate solution, what is the color of the deposited substance on the iron nails?
a) Blue
b) Green
c) Brown
d) Red
Answer: c) Brown
Question 4. What happens to the intensity of the blue color in the copper sulphate solution after the reaction with iron nails?
a) It becomes darker
b) It becomes lighter
c) It remains the same
d) It changes to green
Answer: b) It becomes lighter
Question 5. In the reaction between iron nails and copper sulphate solution, which element is displaced by the other?
a) Iron is displaced by copper
b) Copper is displaced by iron
c) Sulphate is displaced by iron
d) Sulphate is displaced by copper
Answer: b) Copper is displaced by iron