Quantum numbers are numerical values that describe the properties of atomic orbitals and the electrons in those orbitals. They are essential for understanding the arrangement of electrons in atoms and the resulting chemical behavior of elements. There are four primary quantum numbers: principal (n), azimuthal (l), magnetic ($m_l$), and spin ($m_s$).
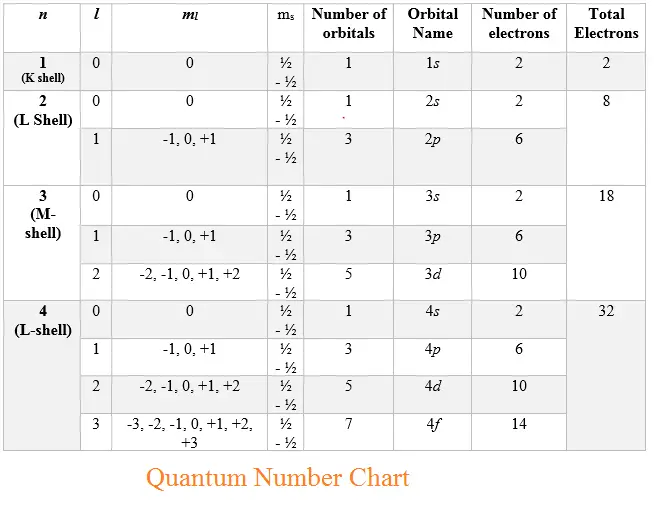
Here is the chart of allowed Quantum numbers
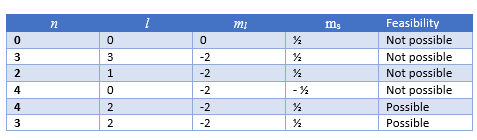
We can easily see the below combinations are not possible for Quantum numbers
- Principal Quantum Number determines the energy level and size of the orbital. Higher ? n values correspond to orbitals further from the nucleus.
- Azimuthal Quantum Number determines the shape of the electron cloud and the number of angular nodes.
- Magnetic Quantum Number determines the number of orbitals and their orientation within a given subshell.
- Spin Quantum Number specifies the two possible spin states of an electron in an orbital, often referred to as “spin up” and “spin down.”
Related Articles
Quantum number worksheet
How to Find Quantum Numbers for an atom
Quantum Mechanical Model
Electronic Configuration of Elements 1-30

It is proposed here to represent the quantum distribution of atomic orbitals in an unprecedented table where the quantum shells and subshells are drawn in the form of chevrons whose vertices are occupied by orbitals with the magnetic quantum number m = 0. This new representation visually shows, much better than a classic linear chart, the relationship between the number of quantum shells and the number of orbitals.
https://www.researchgate.net/publication/346080523_Chevron_Form_Quantum_Charts
Thanks ? I’m a high school Chemistry teacher and in process of exam writing for grade 12 I saw your notes.