Here is CBSE class 11 Chemistry Syllabus 2024-2025
CLASS XI (THEORY)
(Total Periods 110)
Unit I: Some Basic Concepts of Chemistry (Periods 12)
General Introduction: Importance and scope of Chemistry. Nature of matter, laws of chemical combination, Dalton’s atomic theory: the concept of elements, atoms and molecules. Atomic and molecular masses, mole concept and molar mass, percentage composition, empirical and molecular formula, chemical reactions, stoichiometry and calculations based on stoichiometry.
Unit II: Structure of Atom (Periods 14)
Discovery of electron, proton and neutron; atomic number, isotopes and isobars. Thompson’s model and its limitations, Rutherford’s model and its limitations, Bohr’s model and its limitations, concept of shells and subshells, dual nature of matter and light, de Broglie’s relationship, Heisenberg uncertainty principle, concept of orbitals, quantum numbers, shapes of s, p and d orbitals, rules for filling electrons in orbitals – Aufbau principle, Pauli exclusion principle and Hund’s rule, electronic configuration of atoms, stability of half-filled and completely filled orbitals.
Unit III: Classification of Elements and Periodicity in Properties
(Periods 8)
Significance of classification, brief history of the development of periodic table, modern periodic law and the present form of periodic table, periodic trends in properties of elements –atomic radii, ionic radii, inert gas radii, ionization enthalpy, electron gain enthalpy, electronegativity, valence. Nomenclature of elements with atomic number greater than 100.
Unit IV: Chemical Bonding and Molecular Structure (Periods 14)
Valence electrons, ionic bond, covalent bond, bond parameters, Lewis structure, polar character of covalent bond, covalent character of ionic bond, valence bond theory, resonance, geometry of covalent molecules, VSEPR theory, concept of hybridization involving s, p and d orbitals and shapes of some simple molecules, molecular orbital theory of homonuclear diatomic molecules (qualitative idea only). Hydrogen bond.
Unit VI: Chemical Thermodynamics 16 Periods
Concepts of System and types of systems, surroundings, work, heat, energy, extensive and intensive properties, state functions. First law of thermodynamics -internal energy and enthalpy, heat capacity and specific heat, measurement of ?U and ?H, Hess’s law of constant heat summation, enthalpy of bond dissociation, combustion, formation, atomization, sublimation, phase transition, ionization, solution and dilution. Second law of Thermodynamics (brief introduction) Introduction of entropy as a state function, Gibb’s energy change for spontaneous and non- spontaneous processes, criteria for equilibrium. Third law of thermodynamics (brief introduction).
Unit VII: Equilibrium (Periods 14)
Equilibrium in physical and chemical processes, dynamic nature of equilibrium, law of mass action, equilibrium constant, factors affecting equilibrium – Le Chatelier’s principle; ionic equilibrium – ionization of acids and bases, strong and weak electrolytes, degree of ionization, ionization of polybasic acids, acid strength, concept of pH, Hydrolysis of salts (elementary idea), , buffer solutions, Henderson equation, solubility product, common ion effect (with illustrative examples).
Unit VIII : Redox Reactions (Periods 6)
Concept of oxidation and reduction, redox reactions, oxidation number, balancing redox reactions incterms of loss and gain of electron and change in oxidation numbers , applications of redox reactions.
Unit XII: Organic Chemistry – Some Basic Principles and Techniques( 14 Periods)
General introduction, methods of purification, qualitative and quantitative analysis, classification and IUPAC nomenclature of organic compounds. Electronic displacements in a covalent bond: inductive effect, electromeric effect, resonance and hyper conjugation.
Homolytic and heterolytic fission of a covalent bond: free radicals, carbocations, carbanions, electrophiles and nucleophiles, types of organic reactions.
Unit XIII: Hydrocarbons (Periods 12)
Classification of Hydrocarbons
Aliphatic Hydrocarbons:
Alkanes – Nomenclature, isomerism, conformation (ethane only), physical properties, chemical reactions including free radical mechanism of halogenation, combustion and pyrolysis.
Alkenes – Nomenclature, the structure of double bond (ethene), geometrical isomerism, physical properties, methods of preparation, chemical reactions: addition of hydrogen, halogen, water, hydrogen halides (Markovnikov’s addition and peroxide effect), ozonolysis, oxidation, mechanism of electrophilic addition.
Alkynes – Nomenclature, the structure of triple bond (ethyne), physical properties, methods of preparation, chemical reactions: acidic character of alkynes, addition reaction of – hydrogen, halogens, hydrogen halides and water.
Aromatic Hydrocarbons:
Introduction, IUPAC nomenclature, benzene: resonance, aromaticity, chemical properties: mechanism of electrophilic substitution. Nitration, sulphonation, halogenation, Friedel Craft’s alkylation and acylation, directive influence of the functional group in monosubstituted benzene. Carcinogenicity and toxicity.
PRACTICALS
Total Periods 60
Micro-chemical methods are available for several of the practical experiments. Wherever possible such techniques should be used.
A) Basic Laboratory Techniques (Periods 2)
Cutting glass tube and glass rod
Bending a glass tube
Drawing out a glass jet
Boring a cork
B) Characterization and Purification of Chemical Substance
(Periods 6)
- Determination of melting point of an organic compound.
- 2. Determination of boiling point of an organic compound.
Crystallization involving impure sample of any one of the following:
Alum, copper sulphate, Benzoic acid.
C) Experiments Related to pH Change (Periods 6)
(a) Any one of the following experiments:
- Determination of pH of some solutions obtained from fruit juices, solutions of known and
varied concentrations of acids, bases and salts using pH paper or universal indicator.
- Comparing the pH of solutions of strong and weak acid of same concentration.
- Study the pH change in the titration of a strong acid with a strong base using universal indicator.
( b) Study of pH change by common-ion effect in case of weak acids and weak bases.
D) Chemical Equilibrium (Periods 4)
One of the following experiments:
(a) Study the shift in equilibrium between ferric ions and thiocynate ions by increasing /decreasing the concentration of either of the ions.
(b) Study the shift in equilibrium between [Co (H2O)6]2+and chloride ions by changing the concentration of either of the ions .
E) Quantitative Estimation (Periods 16)
- Using a chemical balance.
- Preparation of standard solution of oxalic acid.
- Determination of strength of a given solution of sodium hydroxide by titrating it against standard solution of oxalic acid.
- Preparation of standard solution of sodium carbonate.
- Determination of strength of a given solution of hydrochloric acid by titrating it against standard
sodium carbonate solution.
F) Qualitative Analysis (Periods 16)
(a) Determination of one anion and one cation in a given salt
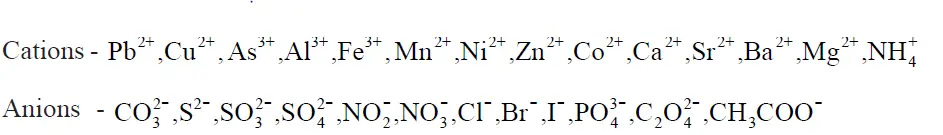
(Note : Insoluble salts excluded)
(b) Detection of nitrogen, sulphur, chlorine, in organic compounds.
Project (Periods 10)
Scientific investigations involving laboratory testing and collecting information from other sources.
A few suggested Projects
• Checking the bacterial contamination in drinking water by testing sulphide ion
• Study of the methods of purification of water
• Testing the hardness, presence of Iron, Fluoride, Chloride, etc., depending upon the regional variation in drinking water and study of causes of presence of these ions above permissible limit (if any).
• Investigation of the foaming capacity of different washing soaps and the effect of addition of Sodium carbonate on it
• Study the acidity of different samples of tea leaves.
• Determination of the rate of evaporation of different liquids.
• Study the effect of acids and bases on the tensile strength of fibers.
• Study of acidity of fruit and vegetable juices
Note: Any other investigatory project, which involves about 10 periods of work, can be chosen with the approval of the teacher.
You Can download this from below Link
Download CBSE Class 11 Chemistry Syllabus
Useful Links for Class 11 Physics
Full study Material with questions
Useful Text Books for Class 11 Physics
- Principles of Physics Extended (International Student Version) (WSE)
- university physics with modern physics by Hugh D. Young 13th edition
- NCERT Exemplar Problems: Solutions Physics Class 11
- CBSE All in One Physics Class 11 by Arihant
- NCERT Solutions: Physics Class 11th
- New Simplified Physics: A Reference Book for Class 11 (Set of 2 Parts)
- Pradeep’s A Text Book of Physics with Value Based Questions – Class XI (Set of 2 Volumes)
- Oswaal CBSE Sample Question Papers For Class 11 Physics (For 2016 Exams)
Useful CBSE links
