Calcium is denoted by the letter “Ca” and its atomic number is 20. The number of electrons in Calcium is 20. We will checking about electron configuration of calcium
The electron configuration of calcium
Electron configuration is the arrangement of electrons in different orbitals of an atom. It is obtained as per the below rules
(a) As per the Aufbau rule or Principle, the orbitals are filled in increasing order of their energies.
1s, 2s ,2p, 3s ,3p ,4s ,3d ,4p, 5s,4d,5p,4f,5d,6p
(b) There can be a maximum of two electrons in the orbital
(c) the pairing of electrons in the orbitals belonging to the same subshell [ p, d, f], does not take place until each orbital gets singly occupied
Based on the above rules, The electronic configuration of Calcium is given by
1s2 , 2s2, 2p6,3s2,3p6,4s2
or
2, 8,8,2
So it is not like 2,8,10 or 2,8,9,1 as you might think
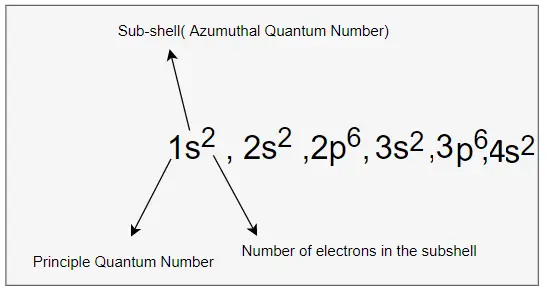
we can write the electron configuration in the orbital diagram as below

Sometimes, we can write the electron configuration in short form as
[Ar] 4s2
Where [Ar] stands for electron configuration of Argon i.e 1s2 , 2s2, 2p6,3s2,3p6
I hope you like this article on Electron configuration of calcium
Related Articles
Electron configuration of oxygen
Electron configuration of sodium
Electronic Configuration
