Sodium is denoted by the letter Na and its atomic number is 11. The number of electrons in Sodium is 11. This post we will checking Electron configuration of sodium and Electron configuration of sodium ion
Electron configuration of Sodium
Electron configuration is the Arrangement of electrons in different orbitals of an atom. The electronic configuration of sodium is given by
1s2 , 2s2, 2p6 ,3s1
or
2, 8,1
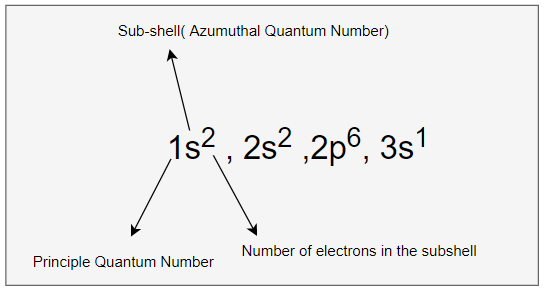
we can write the electron configuration in the orbital diagram as below
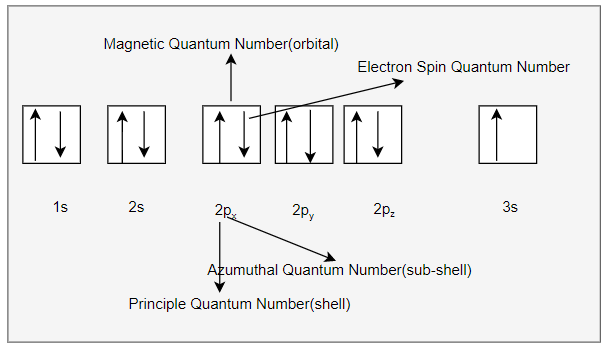
Sometimes, we can write the electron configuration in short form as
[Ne] 3s1
Where [Ne] stands for electron configuration of Neon i.e 1s2 , 2s2, 2p6
Electron configuration of Sodium ion
- The sodium ion is formed by losing 1 electron. The number of electrons in Sodium Ion is 10
- The electronic configuration of Sodium Ion is given by
1s2 , 2s2, 2p6
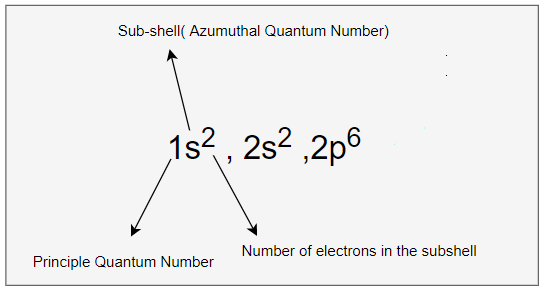
we can write the electron configuration in the orbital diagram as below
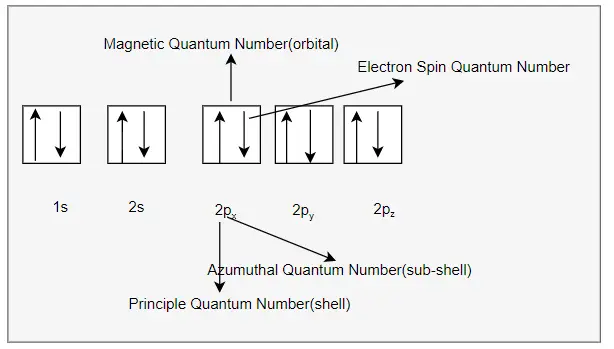
I hope you like this article on Electron configuration of sodium
Related Articles
Electron configuration of oxygen
Electronic Configuration
Electron configuration of calcium
