what is olfactory indicator?
Olfactory indicators are natural substances that can detect acidic or basic solutions based on changes in their characteristic smell or odor. These indicators work by having different odors in acidic and basic media, making them useful for identifying the nature of unknown solutions without using traditional color-changing indicators.
Also Read: Activity 2.2 Class 10 science: Exploring Olfactory Indicators
How Olfactory Indicators Work
Olfactory indicators contain organic compounds whose molecular structure changes slightly in different pH environments. When these structural changes occur, the compounds release different aromatic molecules, resulting in distinct smells in acidic versus basic solutions.
Common Examples of Olfactory Indicators
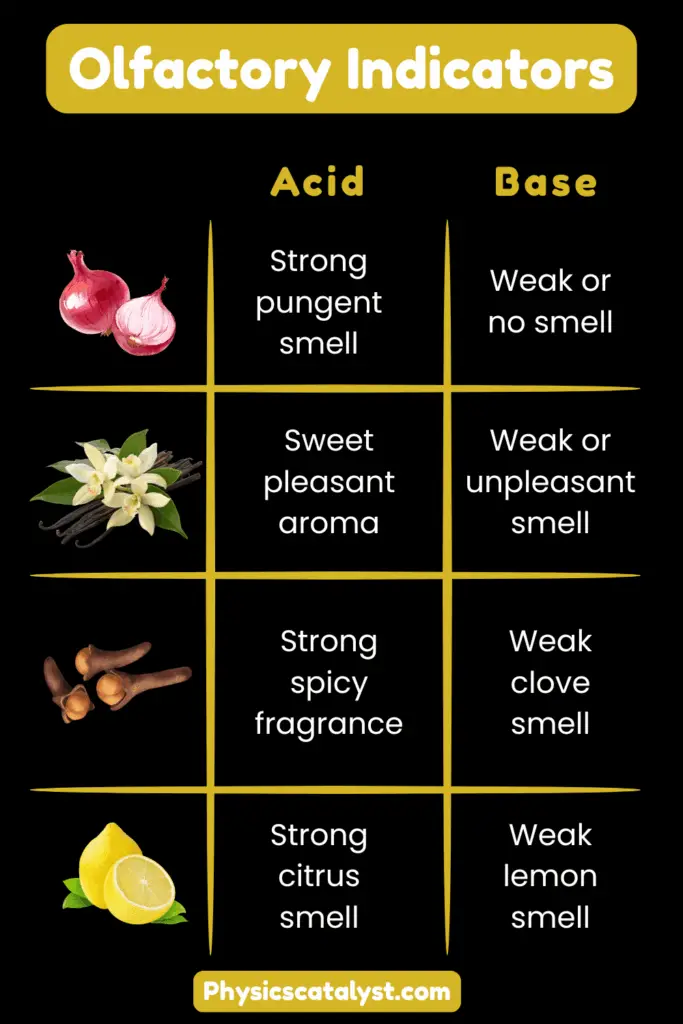
Onion Extract: Fresh onion juice has a characteristic pungent smell. In acidic solutions, this smell remains strong and sharp. However, in basic solutions, the smell becomes much weaker or may disappear completely.
Vanilla Extract: Pure vanilla extract has a sweet, pleasant aroma. In acidic conditions, this pleasant smell is retained. In basic solutions, the vanilla smell diminishes significantly or changes to a less pleasant odor.
Clove Oil: Clove oil possesses a strong, spicy fragrance. In acidic media, it maintains its characteristic clove smell. In basic solutions, the intensity of the clove odor reduces considerably.
Properties and Applications
| Olfactory Indicator | Smell in Acidic Solution | Smell in Basic Solution | Source |
|---|---|---|---|
| Onion Extract | Strong pungent smell | Weak or no smell | Fresh onion juice |
| Vanilla Extract | Sweet pleasant aroma | Weak or unpleasant smell | Vanilla beans |
| Clove Oil | Strong spicy fragrance | Weak clove smell | Clove buds |
| Lemon Extract | Strong citrus smell | Weak lemon smell | Fresh lemon juice |
Practical Applications
Olfactory indicators are particularly useful when:
- Visual observation is difficult due to colored solutions
- Testing solutions that might stain traditional indicators
- Conducting qualitative analysis in field conditions
- Teaching students about different types of chemical indicators
Advantages and Limitations
Advantages:
- Easy to prepare from natural sources
- Cost-effective and readily available
- Useful when visual indicators cannot be used
- Demonstrate the versatility of natural substances in chemistry
Limitations:
- Less precise than pH meters or color indicators
- Subjective interpretation based on individual smell sensitivity
- Cannot provide exact pH values
- Some people may have reduced sense of smell
Summary
Olfactory indicators provide an alternative method for distinguishing between acidic and basic solutions through smell changes. While they are not as precise as conventional indicators, they serve as excellent teaching tools to demonstrate how different senses can be used in chemical analysis and help students understand that indicators work through various mechanisms beyond just color changes.