Although we often use the term “pure substance” informally, it has a precise meaning in science, particularly chemistry. In chemistry, a pure substance has definite composition and distinct chemical properties. We will explore what is pure substances in chemistry in this article.
In everyday language, a pure substance is one that has no contamination or impurities in it. But in chemistry, the term is used in a different or more precise way. In chemistry it is
- made up of only one type of particle
- have a structure that remains fixed
Definition
A pure substance in chemistry is an element or compound comprised entirely of only one type of particle. Pure substances have a definite structure and properties.
Definition
Pure Substance Examples
Some examples of pure substances include
- Gold
- Diamond
- Silver
- Pure water
- Sodium chloride
- Helium
- Bronze
- steel
It is important to note here that the substances mentioned above as examples of pure substances are pure only when there are no impurities in the sample.
For example, water is a pure substance as long as it only has two hydrogen atoms and one oxygen atom. Most of the time in nature, you can’t find water by itself. It usually has other minerals or chemical compounds in it.
Pure substance examples at home
Diamonds, baking soda, sugar, and other pure substances are common in homes. They are pure because they have the same chemical structure throughout.
Examples of impure substances
In general, a heterogeneous mixture with different varieties of substances in it is not a pure substance. From a chemistry point of view, a material is not pure if you can see differences in the composition of the material.
Some of the examples of heterogeneous mixtures or impure substances include
- rocks
- sand
- oil and water
- concrete
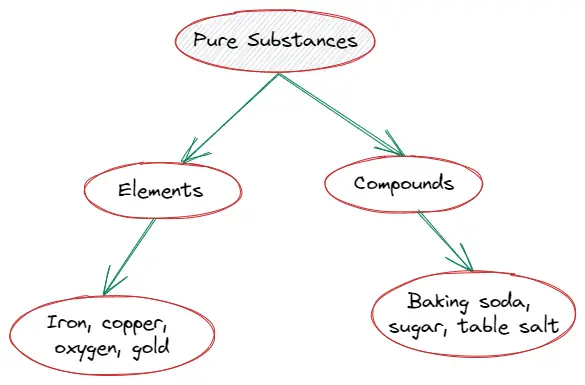
Types of pure substance
Pure substances are categorized into two groups according to their chemical composition. So, there are two types of pure substances :
- Elements
- Compounds
The figure below shows the microscopic structure of both types of pure substances namely elements and compounds. From this, we can clearly see that the composition of both elements and compounds remains throughout the sample.
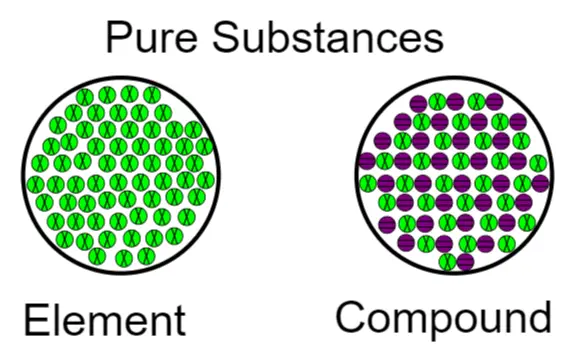
Elements
Let us now learn about elements in chemistry,
- Elements only have one type of atom.
- Elements cannot be physically or chemically separated into two or more simpler substances.
- For example, when pure brass is broken down, you still get brass.
- Elements fall into one of three categories: metalloids, non-metals, or metals.
Compounds
- A compound is a pure substance containing two or more kinds of molecules that have been chemically combined in a specific proportion.
- Compounds have a distinct property that differs from the properties of their constituent elements.
- Physical methods cannot separate the constituents of a compound.
- However, chemical and electrochemical procedures can be used to separate them.
- Electrolysis is a method that can be used for separating hydrogen and oxygen from water.
Properties of pure substance
Let us now have a look at the properties of pure substances.
- Since they are made up of one type of molecule or atom they are generally homogeneous in nature.
- Their composition is uniform throughout.
- These substances have fixed or sharp melting and boiling points.
- The presence of a pure substance in a chemical reaction always results in the creation of predictable products.
You might also like
Frequently asked questions
is helium a pure substance?
Helium is an example of matter and falls under the category of pure substances. It is the second element of the periodic table. It is a pure substance because an element is not formed by mixing two or more substances.
is sugar water homogeneous or heterogeneous?
The sugar in a sugar-water solution is distributed uniformly in the water. As a result, the sugar solution is homogeneous. It is a homogeneous mixture of sugar and water.
is concrete a pure substance?
Concrete is not a pure substance. It’s made up of cement, water, sand, and gravel. This mixture hardens into a stone-like material after some time.
What are impure substances?
Impure substances are created when you mix two or more pure substances in any proportion. Mixtures are impure substances. They can be either homogeneous or heterogeneous mixtures. Air, seawater, petroleum, and a sugar solution in water are all impure substances.