Exploring Acid-Base Reactions: Copper Oxide and Hydrochloric Acid
Introduction
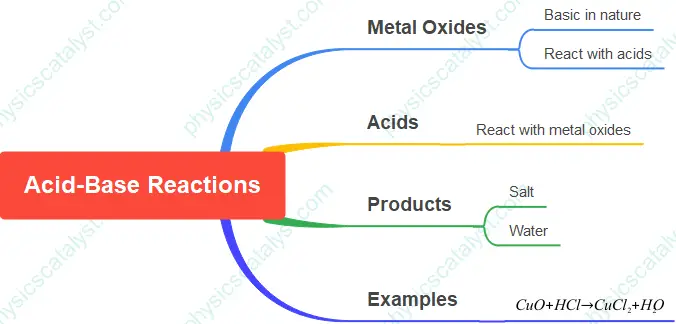
Activity 2.7 from Chapter 2 of the NCERT Class 10 Science textbook demonstrates the reaction between a metal oxide (copper oxide) and an acid (hydrochloric acid). This activity helps students understand the basic nature of metal oxides and their reactions with acids.

Materials Needed
- Copper oxide \(CuO\)
- Dilute hydrochloric acid \(HCl\)
- Beaker
- Glass rod for stirring
Procedure
- Take a small amount of copper oxide in a beaker.
- Slowly add dilute hydrochloric acid to the beaker while stirring the mixture.
- Observe the color change in the solution.
Observations
- The initial color of copper oxide is black.
- As hydrochloric acid is added and stirred, the solution turns blue-green.
- The copper oxide gradually dissolves in the acid.
Explanation
The blue-green color of the solution is due to the formation of copper(II) chloride. This reaction demonstrates that metal oxides react with acids to form salts and water. The general equation for this type of reaction is:
$\text{Metal oxide} + \text{Acid} \rightarrow \text{Salt} + \text{Water}$
In this specific case, the balanced chemical equation is:
${CuO + 2HCl \rightarrow CuCl_2 + H_2O}$
Copper oxide $(CuO)$ reacts with hydrochloric acid $(HCl)$ to produce copper(II) chloride $(CuCl_2)$ and water $(H_2O)$.
Conclusion
This activity demonstrates that metal oxides, such as copper oxide, are basic in nature. They react with acids to form salts and water, similar to the reaction between a base and an acid. Therefore, metal oxides are classified as basic oxides.
Questions and Answers
- Q: Why is copper oxide considered a basic oxide?
A: Copper oxide is considered a basic oxide because it reacts with acids to form salts and water, similar to the reaction of a base with an acid. - Q: What causes the blue-green color of the solution?
A: The blue-green color is due to the formation of copper(II) chloride $(CuCl_2)$ in the reaction. - Q: Write the balanced chemical equation for the reaction between copper oxide and hydrochloric acid.
A: $CuO + 2HCl \rightarrow CuCl_2 + H_2O$ - Q: How would you classify the reaction between copper oxide and hydrochloric acid?
A: This reaction can be classified as a neutralization reaction, where a basic oxide reacts with an acid to form a salt and water. - Q: What would happen if you used a different metal oxide in this experiment?
A: Different metal oxides would react similarly with hydrochloric acid, forming their respective metal chlorides and water. However, the color of the resulting solution may vary depending on the metal involved.
Safety Precautions
- Always wear safety goggles and gloves when handling acids.
- Add acid to water, never water to acid.
- Perform the experiment in a well-ventilated area.
- In case of skin contact with acid, rinse immediately with plenty of water.
Concept Mind Map – Acid-Base Reactions