Activity 2.3 class 10 science: Zinc granule reaction with acid
In this article learn in detail about Activity 2.3 class 10 science, from NCERT book Chapter 2 ‘Acid, Bases and Salts’. This activity teaches students about the reaction of zinc granules with dilute sulphuric acid and the subsequent testing of hydrogen gas by burning.
visit this page for class 10 science notes for physics, chemistry and biology.
Aim (or objective)
The aim of this experiment, Activity 2.3 from the Class 10 Science NCERT book, is to:
- Understand the reaction between a metal (zinc in this case) and an acid (dilute sulphuric acid initially, followed by other acids like $HCl$, $HNO_3$, and $CH_3COOH$).
- Learn how to test for the presence of hydrogen gas using a simple ‘pop’ test.
Theory
The theory behind this activity is based on the chemical reaction between metals and acids. When an acid reacts with a metal, a salt and hydrogen gas are produced. This is a type of single displacement reaction where the metal displaces the hydrogen from the acid to form a salt.
Reaction
In this specific activity, zinc granules are added to dilute sulphuric acid. The zinc displaces the hydrogen from the sulphuric acid to form zinc sulphate, a salt, and hydrogen gas. The chemical equation for this reaction is as follows:
$Zn (s) + H_2SO_4 (aq) \rightarrow ZnSO_4 (aq) + H_2 (g)$
Here:
- $Zn$ is Zinc
- $H_2SO_4$ is Sulphuric acid
- $ZnSO_4$ is Zinc sulphate
- $H_2$ is Hydrogen gas
It is a displacement reaction. Zinc is more reactive than hydrogen and displaces hydrogen from dilute acids.
The hydrogen gas produced in this reaction is then tested by passing it through a soap solution to form bubbles. When a burning candle is brought near these bubbles, they burst with a ‘pop’ sound, confirming the presence of hydrogen gas.
The same reaction occurs when other acids like Hydrochloric acid $(HCl)$, Nitric acid $(HNO_3)$, and Acetic acid $(CH_3COOH)$ are used, with the respective salts being formed.
Material Required
For Activity 2.3 from the Class 10 Science NCERT book, the following materials are required:
- Zinc Granules
- Dilute Sulphuric Acid $(H2SO4)$
- Other Acids
- Test Tube
- Delivery Tube
- Soap Solution
- Candle
- Test Tube Holder
- Safety Goggles and Lab Apron
Experiment Procedure
Here is the detailed procedure for Activity 2.3 from the Class 10 Science NCERT book:
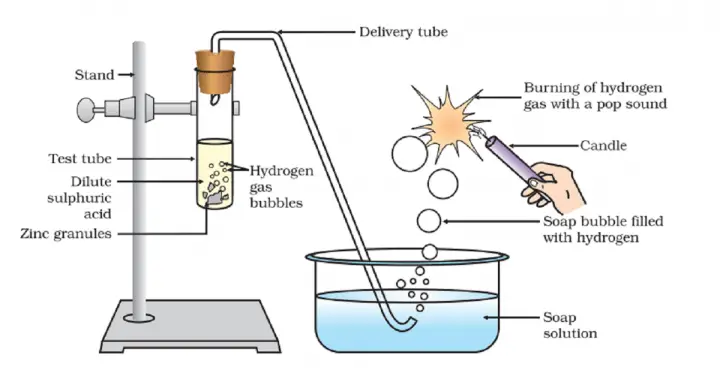
- Setting Up the Apparatus: Arrange the apparatus as shown in the figure given below. This includes a test tube, a delivery tube, a soap solution, and a candle.
- Adding Zinc to Acid: Pour about 5 mL of dilute sulphuric acid into the test tube. Add a few pieces of zinc granules to the test tube containing the acid.
- Observing the Reaction: Watch the surface of the zinc granules. You should see bubbles forming on the surface. This is due to the reaction between the zinc and the sulphuric acid, which produces hydrogen gas.
- Passing the Gas Through Soap Solution: Connect the delivery tube to the test tube and pass the gas being evolved through the soap solution. You will see bubbles forming in the soap solution. These bubbles are filled with the hydrogen gas produced in the reaction.
- Testing for Hydrogen Gas: Bring a burning candle near one of the gas-filled bubbles. The bubble should burst with a ‘pop’ sound, confirming the presence of hydrogen gas.
- Repeating with Other Acids: Repeat the same procedure with other acids like Hydrochloric acid $(HCl)$, Nitric acid $(HNO_3)$, and Acetic acid $(CH_3COOH)$. You should observe similar results, indicating that these acids also produce hydrogen gas when they react with zinc.
Observations
- When zinc granules are added to an acid (like dilute sulphuric acid), bubbles form on the surface of the zinc, indicating the production of hydrogen gas.
- The hydrogen gas, when passed through a soap solution, forms bubbles.
- On bringing a burning candle near a gas-filled bubble, the bubble bursts with a ‘pop’ sound, confirming the presence of hydrogen gas.
Questions and answers based on the activity 2.3 class 10 science
Multiple Choice Questions
- What type of reaction occurs when an acid reacts with a metal?
a) Double displacement reaction
b) Decomposition reaction
c) Single displacement reaction
d) Combination reaction - What happens when zinc granules are added to dilute sulphuric acid?
a) Oxygen gas is produced
b) Hydrogen gas is produced
c) Nothing happens
d) Zinc granules dissolve completely - How is the presence of hydrogen gas confirmed in this activity?
a) By observing the colour change in the solution
b) By the formation of bubbles in the soap solution
c) By the ‘pop’ sound when a burning candle is brought near a gas-filled bubble
d) By the disappearance of zinc granules - What is the product when an acid reacts with a metal?
a) A base and water
b) A salt and water
c) A salt and hydrogen gas
d) A base and hydrogen gas - What observation is made when the gas produced in the reaction is passed through a soap solution?
a) The soap solution changes colour
b) The soap solution becomes hot
c) Bubbles are formed in the soap solution
d) The soap solution becomes cold
Answer:
- c) Single displacement reaction
- b) Hydrogen gas is produced
- c) By the ‘pop’ sound when a burning candle is brought near a gas-filled bubble
- c) A salt and hydrogen gas
- c) Bubbles are formed in the soap solution
Conceptual Questions
- Question: What type of reaction occurs when an acid reacts with a metal?
Answer: A single displacement reaction occurs when an acid reacts with a metal. - Question: What is the gas produced when zinc reacts with an acid?
Answer: The gas produced when zinc reacts with an acid is hydrogen. - Question: How can we test for the presence of hydrogen gas in a reaction?
Answer: The presence of hydrogen gas can be tested by bringing a burning candle near a gas-filled bubble. If the bubble bursts with a ‘pop’ sound, it confirms the presence of hydrogen gas. - Question: What happens when the gas produced in the reaction between zinc and acid is passed through a soap solution?
Answer: When the gas produced in the reaction is passed through a soap solution, bubbles are formed. These bubbles are filled with the hydrogen gas produced in the reaction. - Question: What are the products when an acid reacts with a metal?
Answer: When an acid reacts with a metal, a salt and hydrogen gas are produced.
2.5 aim of the activity