Activity 1.11 class 10 science, from NCERT book Chapter 1 “Chemical Reactions and Equations” introduces the concept of oxidation reactions. The heating of copper powder is the first step in this hands-on experiment. Students witness firsthand the process of oxidation as copper transforms into copper oxide as they participate in this simple yet powerful activity.
The experiment not only helps students visualise a chemical change but also reinforces oxidation reaction theory. Furthermore, it enables students to comprehend and interpret chemical equations, resulting in a more in-depth understanding of chemical reactions and their equations.
Understanding Oxidation Activity 1.11 Class 10 Science
The main aim of NCERT Class 10 Science Chapter 1 Activity 1.11 is to demonstrate the process of oxidation through a simple and effective experiment.
The objective of Activity 1.11 class 10 science:
The objective of Activity 1.11 for class 10 science is to demonstrate and understand the oxidation process through copper powder heating.
Theory
The underlying theory of Activity 1.11 is based on the chemistry concept of oxidation reactions.
In its most basic form, oxidation is the process by which a substance gains oxygen. When the copper powder is heated in this activity, it reacts with the oxygen in the air to form copper oxide. This is an example of an oxidation reaction.
The chemical equation for the reaction is:
\[\text{2Cu (s) + O}_2 \text{(g) ? 2CuO (s)}\]In this reaction:
- Copper $(Cu)$, a solid substance, is the reactant that gets oxidized.
- Oxygen $(O_2)$, a gas, is the oxidizing agent that causes the oxidation of copper.
- Copper Oxide $(CuO)$, a solid, is the product of the reaction.
The change in appearance of copper from a shiny, reddish-brown substance to a dull, black substance indicates the chemical change that has occurred. This colour shift is a direct result of the chemical reaction and represents the formation of copper oxide.
In simple terms, Activity 1.11 provides a hands-on demonstration of the oxidation process, allowing students to comprehend this fundamental concept in a more engaging and hands-on manner.
Materials Required
For Activity 1.11, the materials needed are quite simple and readily accessible. Here’s the list of items you would require:
- Copper Powder: About 1 gram of copper powder is needed. This is the main substance that will undergo the chemical reaction.
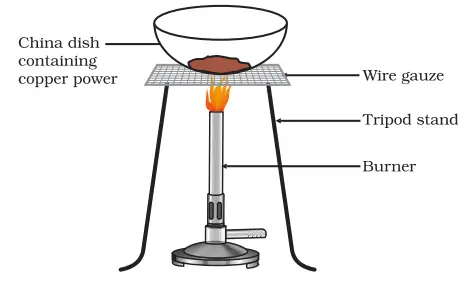
- China Dish: This is used as the container for the copper powder. The china dish can withstand the heat applied during the experiment.
- Heat Source: This could be a Bunsen burner, an electric heater, or a similar device that can heat the copper powder to facilitate the reaction.
- Tongs: These are necessary to handle the heated china dish safely.
When conducting any experiment, always prioritise safety. To ensure a safe and productive learning experience, wear heat-resistant gloves and safety goggles and work in a well-ventilated area.
Experiment Procedure
The experiment starts with a china dish containing 1 gram of copper powder. When this copper powder is heated, a chemical reaction takes place. The following are the steps for carrying out Activity 1.11 for class 10 science:
- Preparation: Collect approximately 1 gram of copper powder. Make sure you have a china dish, a heat source like a Bunsen burner or an electric heater, and tongs for safely handling the hot dish.
- Setting Up: Place the copper powder in the china dish.
- Heating: Heat the china dish containing the copper powder using the heat source. Ensure to do this in a well-ventilated area and maintain a safe distance.
- Observation: Observe the copper powder as it is heated. Note any changes in its colour or appearance.
- Recording Results: Once you notice the copper powder turning black, remove the china dish from the heat source using the tongs. Record your observations and conclude the experiment.
Remember, safety is paramount during any scientific experiment. Always use heat-resistant gloves and safety goggles, and ensure proper ventilation in your workspace.
Observation
The copper powder will change colour significantly as you heat the china dish. Copper has a shiny, reddish-brown colour at first. However, as it is heated, it gradually turns into a dull, black substance. This black substance is nothing more than copper oxide, which is formed when copper reacts with oxygen in the atmosphere.
The Chemical Equation
The chemical process that takes place here can be described by the following equation:
\[\text{2Cu (s) + O}_2 \text{(g) ? 2CuO (s)}\]This equation signifies that when copper (Cu) is heated, it reacts with oxygen (O$_2$) in the air to form copper oxide (CuO). The (s) and (g) denote the physical states of the substances, i.e., solid and gas respectively.
Conclusion
This experiment is an excellent demonstration of an oxidation reaction in which copper is oxidised to form copper oxide. The reaction is an exothermic process that produces heat and results in the observed colour change. As a result, Activity 1.11 demonstrates the basic concept of oxidation in chemical reactions.
