

In this page we have around 40 mcq of acid base and salts class 10 are available for free along with a downloadable pdf of the questions. Acids, Bases, and Salts in CBSE Class 10 Science MCQs contain questions of varied difficulty levels to assist students in preparing for the board test. They can easily get the CBSE Class 10 Science MCQs Chapter 2 Acids Bases and Salts PDF by clicking on the link provided below. They can also obtain CBSE Class 10 Science MCQs for other chapters to help them prepare for the test.
The Acids, Bases, and Salts Class 10 MCQs have been created in accordance with the CBSE Class 10 Science Syllabus and cover the key concepts of Chapter 2. Practising these Class 10 MCQs on Acid Bases and Salts can improve students' preparation so that they may take the board exam with confidence. Students can also practise with the CBSE Class 10 Science Sample Papers based on the most recent exam pattern.
Question 1
When a solution of an acid contains larger amount of acid, it is said to be-
(a) Dilute
(b) concentrated
(c) Mono basic
(d) poly-basic
(b) When a solution of acid contains a larger amount of acid, it is said to be concentrated. Concentrated acid solution has high amount of acid present in less amount of water.
Question 2
Metals like sodium, potassium and calcium react with an acid to liberate-
(a) CO2
(b) NH3
(c) O2
(d) H2
(d) Metals like sodium, potassium and calcium react with an acid to liberate Hydrogen gas $2Na+2HCl\rightarrow 2NaCl+H_2(gas)$
Question 3
Which of the following is a weak acid?
(a) sulphuric acid
(b) hydrochloric acid
(c) acetic acid
(d) nitric acid
(c) Acetic acid is a weak acid. Acetic acid partially dissociates in water. Hence, it is a weak acid.
Question 4
The chemical formula of baking soda is
(a) Na2Co3
(b) Na2Co3.10H2O
(c) NaHCO3
(d) NaOH
(c) Baking soda is Sodium hydrogen bicarbonate. It's chemical formula is $NaHCO_3$
Question 5
Which of the following phenomena occur, when a small amount of acid is added to water?
(a) ionization
(b) Neutralization
(c) Dilution
(d) Salt formation
(a) and (c) Concentrated acid becomes diluted acid when a tiny amount of acid is added to water. This procedure is known as acid dilution. An aqueous solution, often known as a diluted acid, is one in which the acid undergoes ionisation and releases hydronium ions (H+).
Question 6
Salt form during reaction of sulphuric acid with copper
(a) Na2SO4
(b) CuSO4
(c) K2SO4
(d) NH4CI
(b) When Sulphuric acid reacts with Copper then Copper sulphate CuSO4 forms. $Cu+H_2SO_4+H_2$
Question 7
CuSO4. K2SO4 is
(a) acid salt
(b) a mixed salt
(c) normal salt
(d) double salt
(d) CuSO4. K2SO4 is a double salt. A double salt is a crystalline salt having the composition of a mixture of two simple salts but with a different crystal structure from either.
Question 8
The acid present in vinegar
(a) citric acid
(b) tartaric acid
(c) ascorbic acid
(d) acetic acid
(d) In vinegar, acetic acid is present.
Question 9
Acid reacts with ____ of metals to form salt and water.
(a) hydroxide
(b) carbonates
(c) oxides
(d) sulphides
(a) The acids react with hydroxides of metals to form salt and water.
Question 10
Which of these bases is not an alkali?
(a) NaOH
(b) NH4OH
(c) Al (OH)3
(d) all of the are alkalies
(c) Al (OH)3 is not an alkali.
Question 11
Which substance is used to purify water
(a) Ammonium chloride
(b) copper sulphate
(c) chlorine
(d) none of these
(c) chlorine
Question 12
H3PO4 is the example of-
(a) hydracids
(b) organic acid
(c) oxyacid
(d) salt
(c) oxyacid
Question 13
To protect tooth decay we are advised to brush our teeth regularly. The nature of the tooth paste commonly used is
(a) acidic
(b) neutral
(c) basic
(d) corrosive
(c) basic
Question 14
Which of the following substance will not give carbon dioxide on treatment with dilute acid?
(a) Marble
(b) Limestone
(c) Baking soda
(d) Lime
(d) lime
Question 15
The reaction between an acid and a base to form a salt and water is called ________ reaction.
(a) neutralization
(b) endothermic
(c) both a and b
(d) none of these
(a) It is known as a neutralisation reaction when acids and bases interact because the effects of each other are counteracted. Because energy is produced during this reaction, it is exothermic in nature. These reactions produce salt and water as byproducts.
$Acid+Base\rightarrow Salt+Water$
Hence,the correct answer is option (a).
Question 16
An indicator is what type of compound?
(a) reducing agent
(b) strong base or acid
(c) weak base or acid
(d) salt
(c) Indicators are chemical based compounds used to identify the acidity/alkalinity of a given compound so they should be a very weak acid /base.
Question 17
H2SO4 can be prepared by reaction of water with-
(a) NO2
(b) SO2
(c) N2O
(d) SO3
(d)
Question 18
The acidity of Fe(OH)3 is
(a) 2
(b) 3
(c) 4
(d) 5
(d) ACIDITY OF $Fe(OH)_3$ is : 5 to 8
Question 19
The example of olfactory indicators is
(a) Methyl orange
(b) onion
(c) blue litmus
(d) phenolphthalein
(b) A substance known as an olfactory indicator changes in smell depending on whether it is combined with an acidic or basic solution. Examples include vanilla extract, onion, and clove oil.
Question 20
The aqueous solution of sodium acetate is
(a) basic
(b) neutral
(c) acidic
(d) none of these
(a) Since this reaction produces OH- ions, the sodium acetate solution is basic. $$CH_3COONa\rightarrow CH_3COO^- +Na^+\\H_2O\rightarrow H^+ + OH^-\\ CH_3COONa\rightarrow +H_2O\rightarrow CH_3COOH \text{ (Weak acid)}+ NaOH\text{ (Strong Base)}$$ Unlike $H^+$ ions produced by weak acid $CH_3COOH$, which dissociate quickly, $NaOH$ stays completely dissociated to produce large amounts of $OH^-$ ions. As a result, the solution becomes basic due to the abundance of $OH^-$ ions.
Question 21
Bleaching powder is soluble in cold water giving a milky solution due to
(a) Available chlorine
(b) Lime present in it
(c) Calcium carbonate formation
(d) The absorption of carbon dioxide from atmosphere
(b) Bleaching powder on reaction with cold water form milky solution of calcium hydroxide.
$CaOCl_2+H_2O\rightarrow Ca(OH)_2+Cl_2$
Thus because of the lime present in it, it gives a milky solution when dissolved in water.
Question 22
A blue litmus paper was first dipped in dilute $HCl$ and then in dilute $NaOH$ solution. It was observed that the color of the litmus paper
(a) Changed to red
(b) Changed first to red and then to blue
(c) Changed blue to colorless
(d) Remains blue in both the solutions
(b) Blue litmus turns red in an acid and turns blue in a basic solution. Thus, blue litmus first turns red before turning blue.
Question 23
The acid used in making of vinegar is
(a) Formic acid
(b) Acetic acid
(c) Sulphuric acid
(d) Nitric acid
(b)
Question 24
A solution turns blue litmus red. The PH of the solution is probably
(a) 8
(b) 10
(c) 12
(d) 6
(d) A solution is referred to as acidic if its pH value is less than 7. When a solution's pH is higher than 7, it is considered basic, and when it is equal to 7, it is considered neutral.
Because the litmus changes colour from blue to red when the solution is acidic, the pH of the solution is probably 6. (Less than 7).
Question 25
Reaction of an acid with a base is known as
(a) decomposition
(b) combination
(c) redox reaction
(d) neutralization
(d) When an acid and a base combine, the pH of the resulting solution approaches or equals 7, which denotes a neutral solution.
Question 26
Antacids contains
(a) weak base
(b) weak acid
(c) strong base
(d) strong acid
(a) Because they include magnesium hydroxide, an inherently weak base, antacids are weak bases.
Question 27
Bleaching powder gives smell of chlorine because
(a) is unstable
(b) gives chlorine on exposure to atmosphere
(c) is a mixture of chlorine and slaked lime
(d) contains excess of chlorine
(b) Calcium oxychloride is the main ingredient in bleach. $CaOCl_2$ is its chemical formula.
When bleaching powder and atmospheric carbon dioxide interact, calcium carbonate $(CaCO_3)$ and chlorine gas are produced. When exposed to air, it releases chlorine gas and gives off a chlorine smell. The reaction is $CaOCl_2(s)+CO_2(g)\rightarrow CaC)_3(s)+Cl_2(g)$
Question 28
Plaster of paris is made from
(a) lime stone
(b) slaked lime
(c) quick lime
(d) gypsum
(d)
Question 29
Plaster of Paris hardens by
(a)giving of CO2
(b)changing into CaCO3
(c)combining with water
(d)giving out water
(c)
Question 30
Which of the following is acidic in nature
(a) apple juice
(b) soap solution
(c) slaked lime
(d) lime
(a)
Question 31
Which of the following acid is present in sour milk ?
(a) glycolic acid
(b) lactic acid
(c) citrus acid
(d) tartaric acid
(b)
Question 32
Milk of magnesium is an example of
(a) base
(b) acid
(c) salt
(d) none of these
(a)
Question 33
A solution react with crushed egg- shells to give a gas that turns lime- water milky. The solution contains
(a) NaCl
(b) HCl
(c) LiCl
(d) KCl
(b) The egg shell is made up of Calcium carbonate. Therefore, when it reacts with Hydrochloric acid, it forms Calcium chloride, water, and Carbon dioxide.
Question 34
Which of the following is the weak acid
(a) sulphuric acid
(b) hydrochloric acid
(c) acetic acid
(d) nitric acid
(c) Acetic acid (found in vinegar) is a very common weak acid.
Question 35
Acidity of ammonium hydroxide is
(a) 1
(b) 2
(c) 4
(d) 3
(a) The number of Hydroxide $(OH^-)$ ions that a base can release when dissolved in water is referred to as the acidity of a base $(H_2O)$. For instance, sodium hydroxide, also known as Caustic soda (NaOH), has an acidity of 1. Ammonium hydroxide's chemical name is $NH_4OH$. One $OH^-$ ion is released when $NH_4OH$ is dissolved in water. As a result, $NH_4OH$ has a pH of 1.
Question 36
Which of the following is not an acidic salt
(a)CuSO4
(b)Na2CO3
(c)ZnSO4
(d)NH4NO3
(b)
Question 37
Basic salts are formed by neutralization of
(a)strong acid and strong base
(b)strong acid and weak base
(c)weak acid and weak base
(d)strong base and weak acid
(d)
Question 38
Plaster of paris is obtained
(a)by adding water to calcium sulphate
(b)by adding sulphuric acid to calcium hydroxide
(c)by heating gypsum to a very high temperature
(d)by heating gypsum to 373 K.
(d)
Question 39
When we heat the carbonates of some metals, we get
(a) SO2
(b) H2
(c) CO2
(d) NO2
(c)
Question 40
The chemical formula of caustic potash is
(a) $NaOH$
(b) $Ca(OH)_2$
(c) $NH_4OH$
(d) $KOH$
(d)
Question 41
The table given below shows the reaction of a few elements with acids and bases to evolve Hydrogen gas.
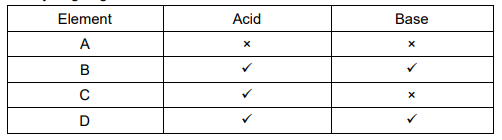
(a) A and D
(b) B and D
(c) A and C
(d) C and D
(b)
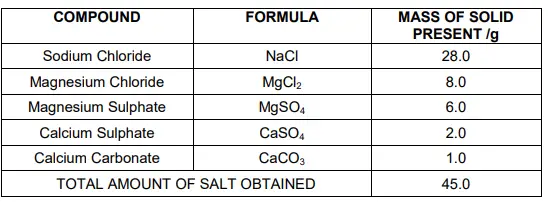
Question 42
Which compound in the table reacts with acids to release carbon dioxide?
(a)NaCl
(b)CaSO4
(c)CaCO3
(d)MgSO4
(c)
Question 43
How many grams of Magnesium Sulphate are present in 180g of solid left by evaporation of sea water?
(a)12g
(b)6g
(c)24g
(d)18g
(c)
Question 44
What is the saturated solution of Sodium Chloride called?
(a)Brine
(b)Slaked lime
(c)Soda water
(d)Lime water
(a)
Question 45
What is the pH of the acid which is used in the formation of common salt??
(a)Between 1 and 3
(b)Between 3 and 7
(c)Between 7 and 11
(d)Between 7 and 14
(a)