Activity 1.1: Ncert book Science class 10
Activity 1.1 from the Class 10 Science NCERT book focuses on the properties and behavior of magnesium when burned in the presence of oxygen. The activity demonstrates a fundamental chemical reaction. This article discusses the process, observations, and conclusions drawn from the experiment.
Understanding the Burning of Magnesium Ribbon in Air and Formation of Magnesium Oxide
Materials and Preparation
To perform this activity, the following materials are required:
- Magnesium ribbon (3-4 cm long)
- Sandpaper
- Tongs
- Spirit lamp or burner
- Watch-glass
Before starting the experiment, clean the magnesium ribbon by rubbing it with sandpaper to remove any oxide layer on its surface. This step ensures that the magnesium reacts efficiently with the oxygen in the air.
Procedure
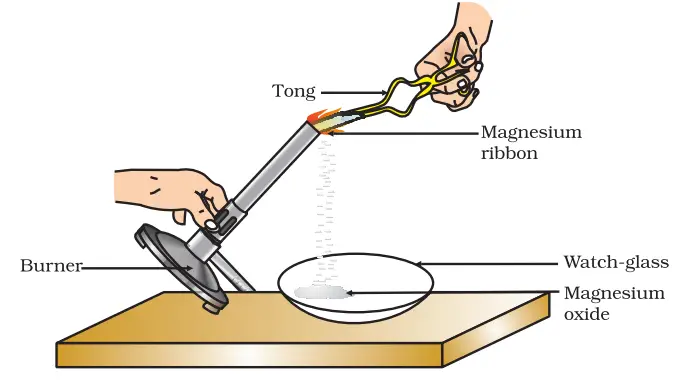
- Hold the cleaned magnesium ribbon with a pair of tongs.
- Ignite the spirit lamp or burner.
- Slowly bring the magnesium ribbon close to the flame, ensuring that it is as far away from your eyes as possible. Exercise caution as the burning magnesium produces a bright, intense light that can be harmful to your eyes.
- Once the magnesium ribbon starts burning, hold it over a watch-glass to collect the ash formed as a result of the reaction.
- Observe the changes that occur during the burning process.
Observations
Upon burning the magnesium ribbon, students will notice the following:
- The magnesium ribbon burns with a dazzling white light, producing a significant amount of heat.
- The ash formed during the burning process is white, powdery, and has a different appearance than the original magnesium ribbon.
- The mass of the ash (magnesium oxide) is slightly higher than the mass of the original magnesium ribbon.
Analysis and Conclusion
The burning of the magnesium ribbon in the presence of oxygen demonstrates a chemical change, specifically a combustion reaction. The intense light and heat produced during the reaction are indicators of the energy released as the magnesium combines with oxygen to form magnesium oxide. The chemical equation for this reaction is as follows:
$2Mg (s) + O_2 (g) \rightarrow 2MgO (s)$
The ash collected in the watch-glass is magnesium oxide (MgO), a compound formed from the combination of magnesium and oxygen atoms. The increase in mass of the ash compared to the original magnesium ribbon is due to the added mass of the oxygen atoms that have combined with the magnesium atoms during the reaction.
In conclusion, Activity 1.1 for Class 10 Science demonstrates the combustion reaction of magnesium in the presence of oxygen, resulting in the formation of magnesium oxide. This experiment serves as an introduction to chemical reactions and reinforces the concept of chemical changes. Students gain a better understanding of the properties of magnesium, the role of oxygen in combustion reactions, and the process of forming new compounds.

Make short activities