Exploring Acid-Base Reactions: Activity 2.5 from Class 10 NCERT Science
Introduction
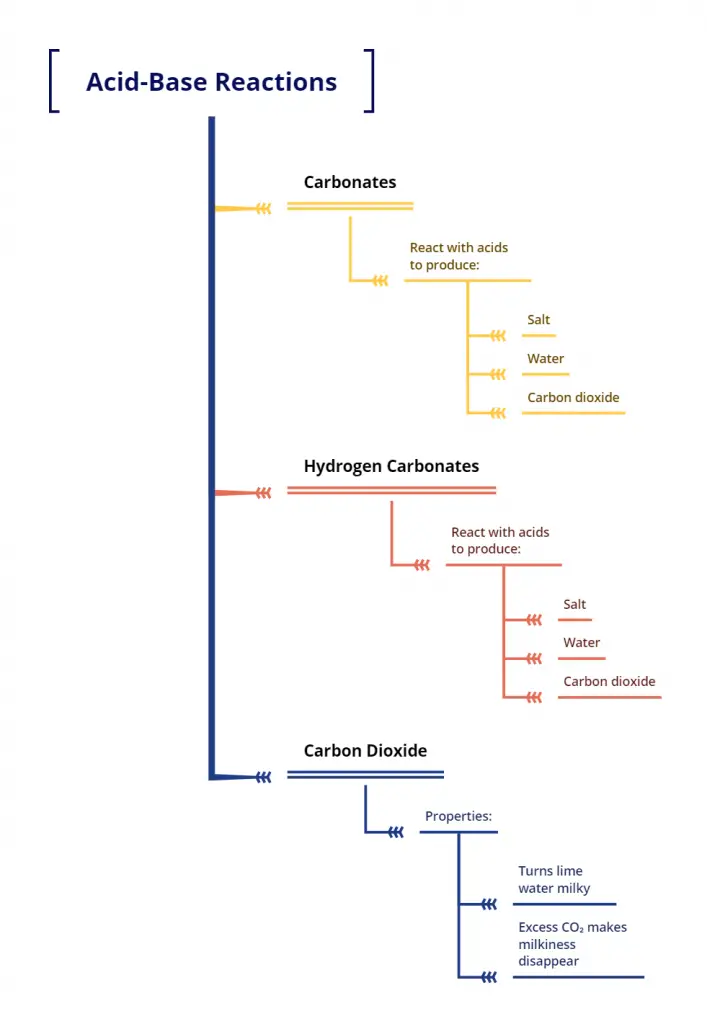
Activity 2.5 from the Class 10 NCERT Science book is an engaging experiment that demonstrates the reactions of carbonates and hydrogencarbonates with acids. This activity belongs to the chapter on Acids, Bases, and Salts, and helps students understand the formation of carbon dioxide gas and its properties.

Materials Needed
- Two test tubes
- Sodium carbonate $(Na_2CO_3)$
- Sodium hydrogencarbonate $(NaHCO_3)$
- Dilute hydrochloric acid $(HCl)$
- Lime water (calcium hydroxide solution)
- Delivery tube
- Test tube holder
- Labels
Procedure
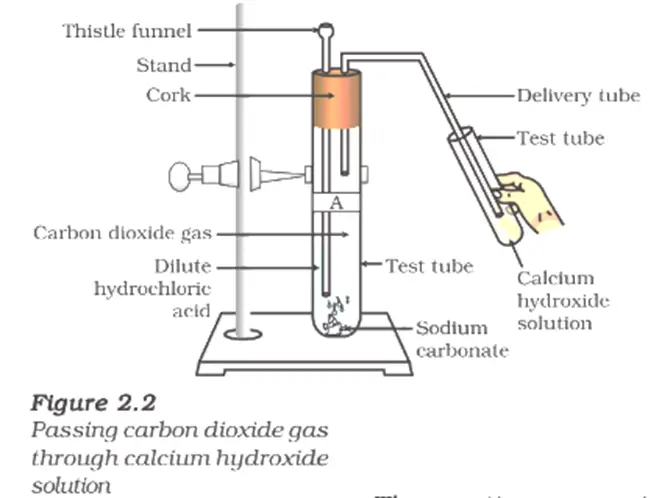
- Label two test tubes as A and B.
- Add about 0.5 g of sodium carbonate $(Na_2CO_3)$ to test tube A.
- Add about 0.5 g of sodium hydrogencarbonate $(NaHCO_3)$ to test tube B.
- Add approximately 2 mL of dilute HCl to both test tubes.
- Observe the reactions in both test tubes.
- Pass the gas produced in each case through lime water using a delivery tube.
- Record your observations.
Observations
- In both test tubes, effervescence (bubbling) is observed when HCl is added.
- When the gas is passed through lime water, it turns milky white in both cases.
Explanation of Chemical Reactions
Test Tube A (Sodium Carbonate)
$$\text{Na}_2\text{CO}_3 (\text{s}) + 2\text{HCl} (\text{aq}) \rightarrow 2\text{NaCl} (\text{aq}) + \text{H}_2\text{O} (\text{l}) + \text{CO}_2 (\text{g})$$
Test Tube B (Sodium Hydrogencarbonate)
$$\text{NaHCO}_3 (\text{s}) + \text{HCl} (\text{aq}) \rightarrow \text{NaCl} (\text{aq}) + \text{H}_2\text{O} (\text{l}) + \text{CO}_2 (\text{g})$$
Lime Water Test
$$\text{Ca(OH)}_2 (\text{aq}) + \text{CO}_2 (\text{g}) \rightarrow \text{CaCO}_3 (\text{s}) + \text{H}_2\text{O} (\text{l})$$
The white precipitate $(CaCO_3)$ causes the lime water to turn milky.
With excess $CO_2$:
$$\text{CaCO}_3 (\text{s}) + \text{H}_2\text{O} (\text{l}) + \text{CO}_2 (\text{g}) \rightarrow \text{Ca(HCO}_3)_2 (\text{aq})$$
This reaction explains why the milkiness disappears with excess $CO_2$.
Conclusion
This activity demonstrates that:
- Carbonates and hydrogencarbonates react with acids to produce carbon dioxide gas.
- $CO_2$ can be identified by its ability to turn lime water milky.
- Excess $CO_2$ can cause the milkiness to disappear due to the formation of calcium hydrogencarbonate.
Questions and Answers
- Q: Why does effervescence occur when HCl is added to both test tubes?
A: Effervescence occurs due to the rapid release of carbon dioxide gas during the reaction. - Q: What is the purpose of passing the gas through lime water?
A: Lime water is used to identify the presence of carbon dioxide gas, as it turns milky white when $CO_2$is bubbled through it. - Q: Why does the milkiness disappear if excess $CO_2$ is passed through lime water?
A: Excess $CO_2$ reacts with the calcium carbonate precipitate to form soluble calcium hydrogencarbonate. - Q: How would you differentiate between a carbonate and a hydrogencarbonate based on this experiment?
A: While both produce $CO_2$, carbonates react more vigorously and produce more gas compared to hydrogencarbonates.
Safety Precautions
- Handle dilute HCl with care, as it is corrosive.
- Wear safety goggles and gloves during the experiment.
- Perform the experiment in a well-ventilated area.
Mind map: Acid-Base Reactions