Activity 1.3: Ncert book Science class 10
This Activity 1.3 in the Class 10 Science NCERT Book Chapter 1 provides a hands-on opportunity for students to explore and observe a chemical reaction between zinc and dilute acids, specifically hydrochloric acid or sulfuric acid. In this article, we will try to understand this activity.
Activity 1.3: Formation of Hydrogen Gas by the Action of Dilute Acids on Zinc
The objective of Activity 1.3 class 10 science:
To observe and analyze the chemical reaction between zinc granules and dilute hydrochloric acid or sulfuric acid.
Materials Required:
- Zinc granules
- Conical flask or test tube
- Dilute hydrochloric acid or sulfuric acid
- Safety goggles and gloves
Procedure:
- Place a few zinc granules into the conical flask or test tube.
- Carefully add dilute hydrochloric acid or sulfuric acid to the container containing the zinc granules.
- Observe any changes occurring around the zinc granules.
- Carefully touch the conical flask or test tube to check for changes in temperature.
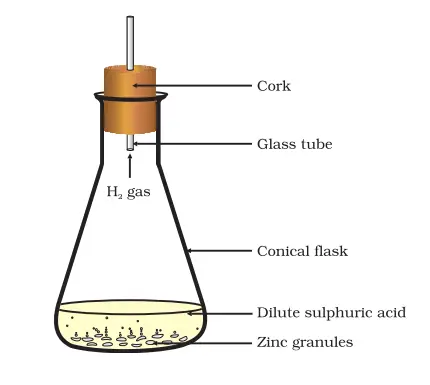
Observations and Analysis:
Upon adding the dilute acid to the zinc granules, students will notice bubbles forming around the zinc granules. This bubbling is an indication of a chemical reaction taking place. The bubbles are formed due to the release of hydrogen gas during the reaction between zinc and the dilute acid.
Additionally, when touching the conical flask or test tube, students will observe a change in temperature, with the container becoming warmer. This increase in temperature is an indication that the chemical reaction occurring is exothermic. An exothermic reaction is one in which energy, in the form of heat, is released to the surroundings.
Chemical Reactions Involved:
The chemical reactions for this activity are as follows:
Zinc + Dilute Hydrochloric Acid $\rightarrow$ Zinc Chloride + Hydrogen Gas
$Zn (s) + 2HCl (aq) /rightarrow ZnCl_2(aq) + H_2(g)$
Zinc + Dilute Sulfuric Acid $\rightarrow$ Zinc Sulfate + Hydrogen Gas
$Zn (s) + H_SO_4 (aq) \rightarrow ZnSO_4 (aq) + H_2 (g)$
These reactions are examples of single displacement reactions, in which one element replaces another element in a compound. In both cases, zinc displaces hydrogen from the acids, resulting in the formation of hydrogen gas and a new compound – either zinc chloride or zinc sulfate.
Significance and Conclusion:
Activity 1.3 is designed to help students visualize and understand the concepts of chemical reactions and equations. By observing the formation of bubbles and experiencing the change in temperature, students gain first-hand experience of an exothermic single displacement reaction.
This activity highlights several important concepts in chemistry:
- Chemical reactions often result in observable changes, such as the formation of gas bubbles or a change in temperature.
- The formation of hydrogen gas in this experiment exemplifies the practical application of single displacement reactions.
- The temperature change observed in this activity demonstrates the concept of exothermic reactions, where energy is released in the form of heat.

?