Activity 1.4: Ncert book Science class 10
Activity 1.4 of Class 10 Science NCERT book chapter demonstrates an exothermic reaction involving calcium oxide and water, resulting in the formation of slaked lime. This article will help you understand the reaction and answering the question posed in the activity.
Activity 1.4 in Class 10 Science NCERT Book: Experimenting with Calcium Oxide
Materials Required for Activity 1.4 class 10 science
To perform Activity 1.4, you will need the following materials:
- A small amount of calcium oxide (quicklime)
- Water
- A beaker
- Safety goggles and gloves
Procedure
Follow these steps to perform Activity 1.4:
- Place a small amount of calcium oxide (quicklime) in the beaker.
- Slowly add water to the quicklime in the beaker. Observe the reaction carefully.
- Touch the beaker gently to feel any change in temperature.
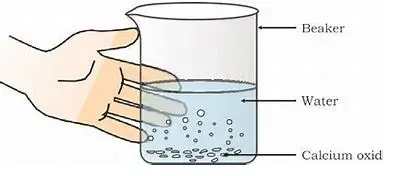
Observations
As you add water to the calcium oxide in the beaker, you will witness a chemical reaction between the two substances. This reaction produces calcium hydroxide, commonly known as slaked lime, and releases heat. The equation for this reaction is as follows:
$CaO (s) + H_2O (l) \rightarrow Ca(OH)_2 (aq)+Heat$
Since heat is released during the reaction, you will feel the beaker getting warmer when you touch it. This indicates an exothermic reaction, where heat is released as a byproduct.
Answering the Question
The question in the activity asks
Whether you feel any change in temperature when you touch the beaker.?
The answer is yes; you will feel the beaker getting warmer due to the exothermic nature of the reaction. The heat released during the reaction causes the temperature change.
Conclusion
This demonstrates the formation of slaked lime by the reaction of calcium oxide with water. This exothermic reaction releases heat, causing a temperature change that can be felt when touching the beaker.
