Activity 1.7 Class 10 Science: Electrolysis of Water
Introduction to Activity 1.7 for class 10 science
Activity 1.7 Class 10 Science in Chapter 1 of the NCERT textbook focuses on demonstrating the process of electrolysis of water. In this hands-on experiment, students will witness the separation of water into its constituent elements, hydrogen, and oxygen, and will be able to identify the gases produced during the process.
The Theory Behind Activity 1.7 class 10 science
Electrolysis is a process in which an electric current is passed through a liquid or solution, causing a chemical reaction to occur.
In the case of Activity 1.7, electrolysis is used to break down water molecules into hydrogen and oxygen gases. The chemical reaction that occurs during electrolysis can be represented as follows:
$2H_2O(l) \rightarrow 2H_2(g) + O-2(g)$
In this reaction, water $(H_2O)$ is the reactant, and hydrogen $(H_2)$ and oxygen $(O_2)$ are the products. This type of chemical reaction is known as decomposition, as it involves the breakdown of a single compound into two simpler substances.
The Role of Dilute Sulphuric Acid
Why is it necessary to add dilute sulphuric acid to the water before initiating the electrolysis process?
In Activity 1.7, dilute sulphuric acid is added to the water before the electrolysis process begins. The purpose of adding the acid is to improve the conductivity of water. Pure water is a poor conductor of electricity because it has a low concentration of ions. By adding a small amount of acid, the concentration of ions in the water increases, making it a better conductor of electricity. The improved conductivity allows the electric current to pass through the solution more easily, facilitating the electrolysis process.
Sulphuric acid $(H_2SO_4)$ dissociates in water, producing hydrogen ions $(H^+)$ and sulfate ions $(SO_4^{2-})$:
$H_2SO_4(aq) \rightarrow 2H+(aq) + SO_4^{2-}(aq)$
These ions increase the conductivity of the water, allowing the electric current to flow more efficiently and promoting the decomposition of water molecules into hydrogen and oxygen gases.
The materials required for Activity 1.7
- Plastic mug with two holes drilled at its base
- Rubber stoppers to fit the holes
- Carbon electrodes
- 6-volt battery
- Water
- Dilute sulphuric acid
- Two test tubes
- Connecting wires
- A burning candle
- Safety goggles and gloves
Setup and Procedure
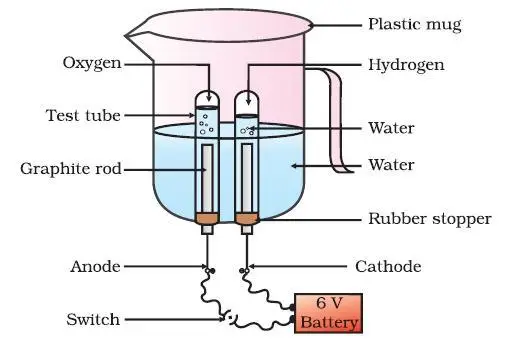
- Preparing the Apparatus: Insert the carbon electrodes into the drilled holes at the base of the plastic mug using rubber stoppers to hold them in place, as shown in Fig. 1.6. Connect the electrodes to a 6-volt battery using connecting wires.
- Preparing the Electrolyte: Fill the plastic mug with water, ensuring that the carbon electrodes are completely immersed. Add a few drops of dilute sulphuric acid to the water to improve its conductivity.
- Collecting the Gases: Fill two test tubes with water and carefully invert them over the carbon electrodes, making sure no air enters the test tubes.
- Initiating the Electrolysis: Switch on the current and leave the apparatus undisturbed for some time. Observe the formation of bubbles at both electrodes, which displace water in the test tubes.
- Observing the Gas Collection: Notice the volume of gas collected in each test tube. Is the volume the same in both test tubes?
- Identifying the Gases: Once the test tubes are filled with the respective gases, remove them carefully, ensuring that no gas escapes. Bring a burning candle close to the mouth of each test tube one by one. Observe the reaction in each case. This step must be performed carefully by the teacher, with students wearing safety goggles and gloves.
Observations and Findings
During the electrolysis process, students will observe that the volume of gas collected in one test tube is approximately double the volume collected in the other test tube. This difference in volume is because water $(H_2O)$ consists of two hydrogen atoms and one oxygen atom, and during electrolysis, the water molecules are broken down into their constituent elements. The test tube with double the volume of gas contains hydrogen, while the other contains oxygen.
When a burning candle is brought near the mouth of the test tubes, the hydrogen-filled test tube will produce a popping sound, indicating the presence of hydrogen gas, which is flammable. In contrast, the oxygen-filled test tube will cause the candle flame to burn more brightly, as oxygen supports combustion.
Conclusion
This activity demonstrates the electrolysis of water, a process that breaks down water molecules into hydrogen and oxygen gases.
Questions related to this activity
Question 1. Explain the process of electrolysis and its significance in Activity 1.7. How does electrolysis lead to the decomposition of water molecules into hydrogen and oxygen gases?
Answer 1. Electrolysis is a process in which an electric current is passed through a liquid or solution, causing a chemical reaction to occur. In Activity 1.7, electrolysis is used to decompose water molecules into hydrogen and oxygen gases. When an electric current is passed through the acidulated water, it facilitates the transfer of electrons, resulting in the breakdown of water molecules as follows:
$2H_2O(l) \rightarrow 2H_2(g) + O_2(g)$
Question 2. During the electrolysis process, different volumes of gases are collected in the two test tubes. Explain the reason for this difference and how it relates to the chemical composition of water.
Answer 2. The difference in the volumes of gases collected in the two test tubes during the electrolysis process is due to the chemical composition of water. Water $(H_2O)$ consists of two hydrogen atoms and one oxygen atom. During electrolysis, water molecules are broken down into their constituent elements, with twice as much hydrogen gas being produced as oxygen gas. This results in a larger volume of hydrogen gas being collected in one test tube compared to the volume of oxygen gas in the other test tube.
Test Yourself
Test yourself for what you have studied in this article. Select the correct options and then submit the test to get results.

thank you this was really helpful, esp the quiz