

Welcome to Class 8 Science chapter 11 notes. On this page, you will find notes, questions, and answers to class 8 science chapter 11. These Chemical effect of electric current Class 8 Notes, explanations, examples, and questions and answers are according to CBSE and the NCERT textbook. If you like the study material, feel free to share the link as much as possible.
Electricity can be defined as a kind of energy formed by moving charges. Metals are considered a good conductor of electricity due to the flow of electric charges in them.
Electric Current-It can be defined as the flow of electrons. An electric current can produce chemical, heating and magnetic effects.
Electric current can not flow on the conductor on its own. An electric circuit, which is a closed-loop path made up of electric components like wire, battery, switch, bulb etc is needed for current to flow in a wire or conductor.
Based on the conductivity, substances are divided into two
1. Conductors –
2. Insulators –
Yes, most liquids can conduct electricity because they have free electrons or ions that carry the electricity through the liquid. Solutions of acids, bases and salts in water conduct electricity and hence are called electrolytes. For example- Lemon juice, sodium hydroxide and Tap water, Milk, Vinegar.
Whereas distilled water doesn’t conduct electricity as it doesn’t contain salts hence distilled water is a bad conductor of electricity. Oil, petrol and kerosene are poor conductors of electricity as they do not have free electrons or ions to conduct electricity.
Note – Milk is a good conductor of electricity because it contains water and lactic acids.
How conductivity of liquids can be tested?
The conductivity of a liquid can be tested by the tester. Testers are used to test whether a particular material allows the electric current to pass through it or not.
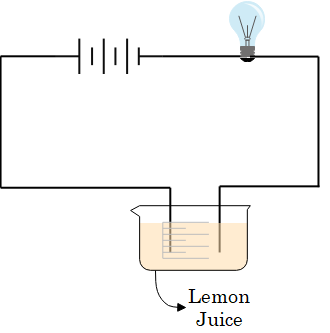

LED bulbs are more suitable for testing the electrical conductivity of liquids. Why?
As the LED bulbs can detect the flow of even a small amount of electric current. The electric current causes a heating effect due to which the filament of the bulb gets heated up and glows. Some liquids are capable of conducting electricity but they are weak conductors of electricity and the current that passes through them are not that strong enough to heat up the filament. But the use of LED bulbs overcome this situation.

Applications of Chemical Effect Of Electric Current
Note- William Nicholson, was the first to discover the chemical effect of current. He discovered that if electrodes were immersed in water, and a current was passed they dissociate into hydrogen ions and oxygen ions.
An electrode is a conductor of electricity that can carry electric current into non-metals and other poor conductors of electricity.
The electrolyte can be defined as the liquids which allow an electric current to pass through them and split themselves on the passage of electric current.
The production or occurrence of chemical change in an electrolyte when an electric current is passed through it. The process of electrolysis shows the following things Depending on the nature of the solution and the electrode.
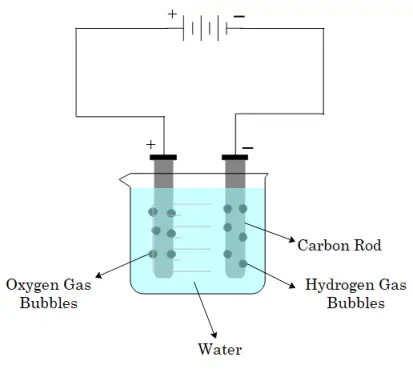
The electrode connected to the negative terminal of the battery is called the cathode (negative electrode).
The electrode connected to the positive terminal of the battery is called an anode (positive electrode).
The process by which a layer of metal is deposited over another metal by the passage of electric current. The kind of metal that is usually electroplated are gold, silver, tin, zinc, copper, chromium.
Electroplating is a very useful process and widely used in industry. Electroplating is a technique for depositing a layer of metal with desired properties on another metal that is utilized in various industries. The main reason is as follows
Electroplating is based on the chemical effects of electricity. Electroplating helps to prevent rusting.
To get the coating of a different metal the following is the process
When an electric current is passed through the copper sulphate solution, copper sulphate dissociates into copper and sulphate. The free copper gets drawn to the electrode connected to the negative terminal (cathode)of the battery and gets deposited on it. The process of electroplating takes some time to complete. The time taken by the process depends upon the strength of the current and also on the concentration of the electrolyte. These two helps to increase the speed of electroplating. We should make sure that the electrode should be clean. The electrodes used are made up of different materials. One of the electrodes is of the same metal of which the electrolyte solution is. The second electrode needs to be the material on which coating takes place.
Electroplating results in the production of harmful chemicals. The disposal of these chemicals is a big concern.
Deflection in compass needle occurs due to the chemical effect of current. True or False
False because deflection in compass needle is a magnetic phenomenon that occurs due to magnetic effects of current.
Which one is a poor conductor?
(a) Acidic solution
(b) Alkaline solution
(c) Common Salt solution
(d) Distilled water
Answer: (d) Distilled water. Conduction of electricity does not take place in distilled water because of the absence of dissolved minerals and salts.
What is Electrolysis?
Electrolysis is the process by which ionic substances are decomposed into simpler substances when an electric current is passed through them.
What is an electric circuit?
A closed-loop path which a current take is known as an electric circuit.
What is the difference between a conductor and an insulator?
Any material that allows an electric current to pass through it is known as a conductor. Metals such as copper, aluminium are good conductors of electricity.
Materials that do not allow an electric current to pass through them easily are called insulators. Insulators are bad conductors of electricity. Some of the examples of insulators are rubber, wood, plastic, glass etc.
What is the difference between distilled water and mineral salt?
Mineral water is water from a mineral spring that contain various minerals and salts. On the other hand, distilled water does not contain salts and minerals.
How does electric current flow through conductors?
The electrons in conductors can move freely and hence carry electric current.
What is Conducting Liquids?
Conducting liquids are those that allow current to flow through them because they contain salt and minerals. Some examples of conducting liquids include lemon Juice, Vinegar, tap water, seawater etc. Conducting liquids are also called electrolytes.
What are the benefits of chromium?
Chrome has a shiny appearance. It does not corrode and is scratch resistant.
What is the difference between iron and tin?
Iron is a more reactive metal than tin.
What is Electroplating?
Electroplating is the process of depositing a layer of any desired metal on another material by means of electricity.