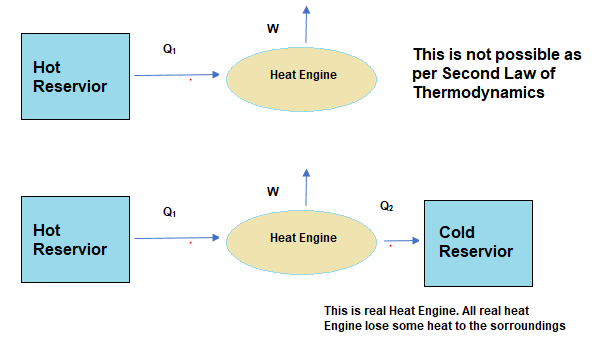
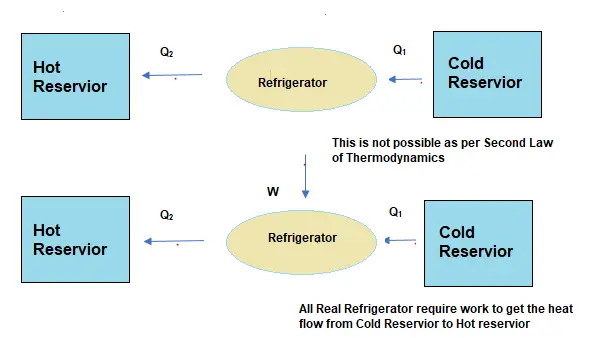
Solved Examples
Question 1
Consider a cycle followed by an engine in below figure
1 to 2 is isothermal
2 to 3 is adiabatic
3 to 1 is adiabatic
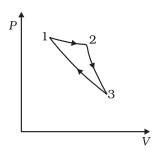
Such a process does not exist because
(a) heat is completely converted to mechanical energy in such a process, which is not possible.
(b) mechanical energy is completely converted to heat in this process,which is not possible.
(c) curves representing two adiabatic processes don't intersect.
(d) curves representing an adiabatic process and an isothermal process don't intersect
Solution
Answer (a) and (c)
Here in this cycle ,we can observe that heat is supplied to the cycle in Isothermal process and there is no heat released in other two process as they are adiabatic Process. So the engine has converted all heat supplied to mechanical work which is not possible as per second law of thermodynamics
We can also prove in the below manner
For Isothermal Process
$\Delta Q_1 =\Delta W_1$
For 2 to 3 adiabatic Process
$\Delta Q_2 =0$ since no heat transfer
$\Delta U_1 =- \Delta W_2$
For 3 to 1 adiabatic Process
$\Delta Q_3 =0$
$\Delta U_2 =- \Delta W_3$
So we can say for whole cycle
$\Delta Q_1 + \Delta Q_2 + \Delta Q_3 =\Delta W_1$
So all the heat supplied is converted into mechanical work which is not possible as per second law of thermodynamics
So (a) is correct
Now (c) is also correct i.e two adiabatic Process cannot intersect as then a cycle can be formed which will violate the second law of thermodynamics